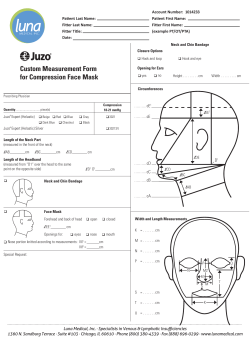
Multimodal treatment strategies for elderly patients with head and
Cancer Treatment Reviews 40 (2014) 465–475 Contents lists available at ScienceDirect Cancer Treatment Reviews journal homepage: www.elsevierhealth.com/journals/ctrv Complications of Treatment Multimodal treatment strategies for elderly patients with head and neck cancer Evangelos G. Sarris a, Kevin J. Harrington b, Muhammad W. Saif c,1, Konstantinos N. Syrigos a,⇑,2 a Oncology Unit GPP, Sotiria General Hospital, Athens Medical School, Building Z, 152 Mesogion Av, 115 27 Athens, Greece The Institute of Cancer Research, London SW3 6JB, UK c Tufts University School of Medicine, Tufts Medical Center, Division of Hematology/Oncology, 800 Washington Street, Box #245, Boston, MA 02111, USA b a r t i c l e i n f o Article history: Received 12 May 2013 Received in revised form 6 October 2013 Accepted 18 October 2013 Keywords: Head and neck cancer Elderly Treatment Multidisciplinary approach Geriatric evaluation a b s t r a c t The population in developed countries is growing older and the number of elderly people annually diagnosed with head and neck cancers is expected to rapidly increase within the following decades, since these types of tumors are age-dependent. The vast majority of older head and neck cancer patients present with locally advanced disease and multimodality treatment, including surgery, radiation and/or chemotherapy, is considered the best therapeutic option for these patients. However, several factors, including comorbidities, disabilities, frailty, and impaired functional status are considered to be more relevant criteria than chronological age per se for treatment planning. Therapeutic decisions are often complicated and demand the participation of many specialists. Advances in surgical and radiation techniques, along with the use of conventional chemotherapy and molecularly targeted agents, have improved treatment outcomes. The best-tailored individualized therapeutic option should be selected for these patients in order to avoid high toxicity and major functional deterioration. Still, more older-specific studies are needed in order to produce more definitive and applicable results. The aim of this review article is to investigate the multimodal treatment approaches for elderly patients with head and neck cancer. Ó 2013 Elsevier Ltd. All rights reserved. Introduction Head and neck cancers (HNC) represent the sixth most common malignancy worldwide and account for approximately 650,000 new cases and 350,000 deaths every year [1]. It was estimated that in the United States alone, approximately 45,660 new cases and 11,210 deaths occurred due to HNC in 2007 [2]. Although the majority of HNC occur between the fifth and sixth decade, almost one-fourth of patients belong in the ‘‘elderly’’ population [3–5]. Especially in developed countries, population is aging and life expectancy is rapidly growing, thus leading to a significant increase in the number of people annually diagnosed with cancer since most types of tumors are age-dependent. Consequently, the incidence of HNC increases with age and geriatric HNC population is expected to rapidly increase within the following decades [6]. In Finland, the percentage of new laryngeal cancer cases diagnosed in patients over 70 years of age in 2006–2007 was 31% both for males and females and for cancers of the mouth and pharynx, 30% and 48% for males and females respectively [7]. ⇑ Corresponding author. Tel.: +30 2107700220; fax: +30 2107781035. E-mail addresses: kevin.harrington@icr.ac.uk (K.J. Harrington), wsaif@tufts medicalcenter.org (M.W. Saif), ksyrigos@med.uoa.gr (K.N. Syrigos). 1 Tel.: +1 (617) 636 2515; fax: +1 (617) 636 8535. 2 Visiting Professor of Thoracic Oncology, Yale School of Medicine, USA. 0305-7372/$ - see front matter Ó 2013 Elsevier Ltd. All rights reserved. http://dx.doi.org/10.1016/j.ctrv.2013.10.007 HNC is a heterogeneous group of aggressive epithelial malignancies that develop in the paranasal sinuses, nasal cavity, oral cavity, pharynx and larynx. The vast majority of HNC are squamous cell carcinomas (SCCHN), for which several risk factors have been identified. Tobacco and alcohol consumption are considered the most important risk factors, although strong evidence exist to support the significant role of human papillomavirus (HPV) as a causal factor in specific subsets of SCCHN. Other risk factors include age, gender, race, previous radiation to the head and neck region, occupational exposure and poor oral hygiene/dental status [8]. Surgery and radiotherapy have long been the cornerstone treatment modalities. Improved surgical and radiation techniques along with the use of systemic agents have improved clinical outcomes in curative therapy. However, approximately two-thirds of HNC patients present with locoregionally advanced disease, while 10% of patients have already metastatic disease at the time of initial presentation [9]. For these patients, a multimodality therapy is required, including surgery, radiation and/or chemotherapy. Treatment decisions are often complicated, and demand the participation of many specialists, including head and neck surgeons, medical oncologists, radiation oncologists, radiologists, plastic surgeons, and dentists. In the literature, the definition of ‘‘elderly’’ is variable. A chronological landmark is considered the age of 70. After this age, an 466 E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 increased incidence of age-related physiological changes is observed, which leads to alterations in pharmacokinetics and pharmacodynamics, resulting in potentially increased treatmentrelated toxicity [10]. The National Institute of Aging and the National Institutes of Health are currently using three categories to define aged patients: 65–74 years as ‘‘young old’’, 75–84 years as ‘‘older old’’ and 85 and over as ‘‘oldest old’’ [11]. Although aging is associated with a variety of declining physiological functions which might affect patient’s ability to withstand cancer treatment, due to the fact that biological age may differ greatly from chronological age, thorough evaluation and risk assessment are needed for decision making. In addition, it is important to estimate the life expectancy of the patient when treatment strategies are planned. In the western societies, the age adjusted life expectancy is about 13.6 years for a 70 year-old male and 16.4 years for a 70 year-old female [10]. Older patients are generally underrepresented, relative to their incidence rates, in most cancer treatment trials [12], while many studies use as exclusion criteria disorders or characteristics that are more common in the elderly (hematologic, renal, cardiac, etc. disorders, functional status limitations, life expectancy, etc.) [13]. This underrepresentation has also been demonstrated in head and neck cancer trials, specifically [13]. Thus, most studies concerning cancer treatment in the elderly are retrospective and subjected to significant selection bias. There are only few Phase III studies having sufficient data for the elderly and they do not always demonstrate beneficial effects for this subgroup of patients. The result is a lack of evidence-based data with regard to the most appropriate treatment, thus depriving them from potential new therapy that may improve their care. Therefore, there is an imperative need for individualized management of the elderly HNC patients in a multidimensional and multidisciplinary way. The aim of this review article is to investigate the multimodal treatment strategies for elderly HNC patients with a view to personalized management. Head and neck cancer in elderly patients: epidemiological data and geriatric evaluation Elderly patients with HNC have some specific features, regarding the clinical presentation of the disease that might differ from those found in younger patients. Oral cavity is the most commonly affected tumor site (46%) in patients aged more than or equal to 80 years, as it was reported by Italiano et al. in a single institution study of 316 HNC patients, while other less common sites are the larynx and oropharynx (23% and 19%) [14]. Other epidemiological studies also verify the significant prevalence of oral cavity cancer in HNC patients aged more than or equal to 70 years [4], whereas a decreased incidence of hypopharyngeal cancer was reported by Sarini et al. in a study of 273 HNC patients aged more than or equal to 74 years [15]. In contrast, data from a non age-specific metaanalysis of 17,346 patients from 93 randomized trials presented oropharyngeal cancer as the most frequently observed (36%), followed by laryngeal and oral cavity cancer (21% each) [16]. Even though HNC is considered a predominantly male disease, several studies have reported a higher women proportion in the elderly HNC population, with a sex ratio close to 1 [4,14,16,17]. In addition, elderly patients with HNC present a significantly lower degree of alcohol and tobacco exposure, and higher rates of comorbidities compared with a group of younger patients [4,17–19], which strengthens the role of advanced age as a risk factor in the development of this malignancy. Increasing age is related with reduced immune surveillance, increased mutation rate and deficient DNA repair mechanisms that may potentially lead to increased cancer incidence [16]. Moreover, elderly HNC patients are more likely to have a second primary cancer compared with younger counterparts (31% vs. 20%) [18]. In a French study of 270 patients with oral cavity cancer aged more than or equal to 80 years, differences in risk factors were also observed between men and women. Tobacco and alcohol consumption were the major risk factors identified in 50% of the male population, whereas chronic oral trauma, leukoplakia and lichen planus were the predominant risk factors found in half of the female population [17]. In addition, HPV infection which is considered a significant causal factor in younger HNC patients seems to have minor effect in the pathogenesis of HNC in the elderly population [20]. However, since HPV status is a significant prognostic factor in the oropharyngeal cancer and HPV positivity is associated with better response to treatment and modality-independent survival benefit [21–23], it should be investigated in any patient with this cancer type, including elderly patients. The majority of elderly HNC patients has locally advanced tumors at the time of initial presentation with, however, less nodal involvement compared with younger patients [18,24,25]. Multimodality treatment including surgery, radiation and/or chemotherapy is considered the best therapeutic option for these patients. However, numerous studies have shown that older HNC patients are less likely to receive potentially curative treatment compared with younger counterparts based on age alone [15,26–29], and in only half of the older HNC patients, therapeutic strategy complies with institution’s policies [17,20,24], although retrospective and prospective data suggest that survival outcomes between older and younger HNC patients are similar [24,30,31]. In addition, a Surveillance, Epidemiology, and End Results (SEER) database analysis of more than 2500 patients with glottic laryngeal, anterior tongue, and tonsillar cancers failed to show any statistically significant difference in overall survival or disease-specific survival between elderly and younger patients after stage stratification [32]. Thus, other factors, including comorbidities, disabilities, frailty, and impaired functional status are considered to be more relevant criteria than chronological age by itself for decision making [33]. Therefore, treatment decisions regarding the elderly HNC population should be rather based on ‘‘functional’’ and not chronological age. The ‘‘functional’’ age of each patient is the most significant parameter for treatment planning and should be defined based on comorbidities and functional status [34,35]. However, comorbidity and functional status should be assessed independently as it was reported by a study in 203 cancer patients with a median age of 75 years using the Cumulative Illness Rating Scale – Geriatric (CIRS-G) and the Charlson score scale [36]. Comorbidity is defined as the presence of additional concurrent illnesses and disorders that are unrelated to HNC. Pulmonary function is decreasing with age due to significant deterioration of the lung parenchyma resulting in reduced vital capacities and impaired gas exchange. In addition, heart function becomes less efficient with age, leading to decreased cardiac output, reduced renal blood flow and greater water and electrolyte imbalances during surgery and general anesthesia. Also, changed renal function and hepatic metabolism may result in different drug distribution in elderly patients [37,38]. Therefore, among HNC populations, comorbidity incidence has a tendency to increase with age [4,39,40]. Other factors, such as race, gender and socioeconomic issues are also related to increased incidence and severity of comorbidity. All these parameters should be carefully evaluated when therapy strategies are planned. Several instruments with the ability to estimate and standardize comorbidity assessment measures, along with quality of life (QOL) measures and symptom assessment tools have been created and validated in HNC [39–41]. Numerous studies have investigated the potential effect of age, comorbidity and/or pre-treatment QOL on prognosis and post-treatment QOL. However, existing data are E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 rather controversial, with some studies showing independent impact of age on disease specific survival [42], others correlate comorbidity with prognosis or QOL [43–47], while other studies highlight the effect of pre-treatment QOL on survival or long-term QOL [43,48]. In addition, studies exist to support no associations between comorbidity and QOL scores [49] or complications [50]. A systematic review of the literature by Paleri et al. concluded that comorbidity increases mortality in HNC patients, and this effect is greater in the early years following treatment. In addition, comorbidity seems to have an impact on disease-specific survival for both older and younger patients, although it is greater in the young-aged group. Higher incidence and increased severity of treatment complications, along with a negative impact on QOL and increased cost of treatment in patients with high comorbidity burden were also reported in the same study [39]. In other studies, increased age and comorbidity incidence negatively influence pretreatment QOL and functional outcomes [43,45,51,52], whereas pre-treatment QOL and comorbidity are associated with poorer post-treatment QOL and survival [43,44,47,48]. On the contrary, a retrospective analysis by Gourin et al. showed no statistically significant association between comorbidity or age and treatment selection or QOL outcomes [49]. Similarly, other studies reported no negative effect of comorbidity on post-treatment QOL [45,50]. Lastly, several studies presented a significant impact of comorbidity on treatment selection, modification and therapy aggressiveness in elderly HNC patients [42,53]. Functional status is another significant factor that should be evaluated when treatment strategies are planned. In a broad term, functional status is measured in terms of performance status evaluation, biological tests (e.g. renal or liver function, molecular markers), frailty measures and measures of activities of daily living [33]. As aforementioned, elderly HNC patients represent a heterogeneous population that includes healthy, common-aging and unfit individuals, thus decisions for diagnosis and treatment are often complex and have to be evaluated in the frame of a multidisciplinary approach including oncogeriatric assessment. Several geriatric instruments have been developed over the last few decades in an effort to evaluate the degree of frailty of these patients. The Comprehensive Geriatric Assessment (CGA) is a multidimensional diagnostic instrument able to evaluate several patients’ characteristics including functional status, comorbidity, emotional status, mental status, nutrition, social support, geriatric syndromes and polypharmacy, in an attempt to highlight elderly patients with increased risk of dismal outcome (Fig. 1). In this way, medical, 467 psychological, and functional status of the elderly patients can be estimated and CGA can be used as a tool for decision-making and patients’ follow-up [54]. The use of CGA along with a multidisciplinary approach will allow oncologists to choose the safest and most effective management for their patients, with a view to personalized patients care [3,55,56]. Vulnerable elders survey-13 (VES-13) is a new function-evaluating tool aiming to identify elderly cancer patients that are more prone to health deterioration. Age, physical status, functional status, and self-rated health status are evaluated in VES-13 in an effort to assess patients before undertaking CGA which is more time consuming [57]. The potential application of VES-13 in the management of HNC patients seems as a promising ground for future research. Balducci classification (three groups of patients based on comorbidities and activities of daily living) is widely used in numerous cancer patients, but it cannot be fully applied in HNC patients due to specific features of these patients. Increased prevalence of malnutrition and highly symptomatic tumors accompanied by rapid functional deterioration, potentially reversible after local treatment, are some of the features that limit the use of Balducci classification in elderly HNC population [58,59]. Treatment modalities in elderly HNC patients Treatment decisions in HNC are often complicated and demand the participation of many specialists. Surgery and radiotherapy have long been the major treatment approaches. However, the complex anatomy and vital physiological role of the cancer-involved structures dictate that primary treatment purposes should include an improved survival outcome-locoregional control but also organ-function preservation. Systemic therapy, including conventional chemotherapy and molecularly targeted agents, could play a potentially curative role in locally advanced tumors. In addition, the management of HNC in a geriatric context is far more complex due to the high toxicity of locoregional treatments and the high risk of functional deterioration in elderly HNC patients with high comorbidity burden and impaired functional status. Therefore, a multidisciplinary approach is essential along with treatment adaptations that will minimize therapy-induced toxicity. The primary treatment goal should be firmly determined (cure or relieve) and the best-tailored individualized therapeutic option should be selected in order to avoid high toxicity and post-treatment significant functional decline. Fig. 1. Comprehensive geriatric assessment in elderly patients with head and neck cancer. 468 E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 Surgery Surgery is a standard treatment for HNC patients especially for early stage resectable malignancies although anatomical extent of the tumor along with the desire to achieve organ preservation could potentially limit its applications. In locally advanced HNC, surgical procedure remains a therapeutic option due to current advances in microsurgical free tissue transfer for reconstruction of surgical defects that allow major reconstructive procedures. When surgical therapy is warranted, there is no evidence to suggest that advanced age alone is a contraindication to ablative and reconstructive surgery, therefore surgery remains an effective treatment modality for both younger and older HNC patients. In elderly HNC patients, surgical procedures are considered feasible and well tolerated with good functional outcomes [60–63], although studies have shown that elders are less likely to undergo surgery compared with younger counterparts [15] However, numerous studies have supported the aggressive surgical approach for the elderly HNC population [29,63,64], reporting similar postoperative complications and mortality rates for both elders and youngers with HNC [29,60]. In patients aged more than or equal to 80 years participating in the French study, the postoperative 30-day mortality rate was as low as 3% [14]. In a retrospective analysis, Clayman et al. compared the outcomes of 43 patients older than 80 years and 79 patients younger than 65 years. In the older group, a higher prevalence of systemic complications was observed, particularly cardiovascular and pulmonary complications, and a higher prevalence of local complications was found in the younger group, even though differences were not statistically significant. Postoperative mortality was 2% in elderly patients and absent in the young-aged group, whereas increased age was found to adversely affect locoregional control (p < 0.001) and disease-specific survival (p = 0.041). Median overall survival was significantly different between the two groups (33% vs. 63%, p < 0.001), but was comparable to an age matched group [29]. Similar data were presented also by Jones et al. in a study in which HNC patients older than 70 years and younger than 66 years participated. No significant differences in perioperative or post-operative complications between the two groups were observed [65]. An aggressive surgical approach to HNC with curative intent could also be considered for elderly patients with locally advanced cancer [64]. In a study of 270 patients with oral cavity cancer aged more than or equal to 80 years, Ortholan et al. compared the efficacy of surgery and radiotherapy in patients with resectable stage III/IV tumors. The disease-free survival and the overall survival were significantly better in the surgical group [17]. However, surgical procedures for locally advanced cancers are more complex, with longer operative time and increased risk of post-operative complications and functional decline. Although age per se is not a prognostic indicator of surgical outcome for major HNC procedures, pre-operative comorbidities evaluated with Charlson score scale or Adult Comorbidity Evaluation-27 (ACE-27) index and time under general anesthesia, are considered prognostic factors for increased post-surgical complications [62,66,67]. Karnofsky performance status, mental status and QOL have been also assessed as prognostic factors in several studies [53]. In a study by Derks et al. in 121 elderly HNC patients (age ranged from 70 to 94 years old), the American Society of Anaesthesiologists (ASA) classification of physical status and pre-operative performance status (PS) were predictive of the post-operative complications incidence. Median survival was 9.6 months in patients aged between 70–79 years, and 5 months in patients aged more than 80 years. Post-operative QOL was similar in both young and elderly age-groups [68]. The predictive role of comorbidities in post-surgical complications was also reported in another study by Sanabria et al. in 242 patients older than 70 years who underwent HNC surgery [69]. Thus, comorbidities assessment should play a significant role in the surgical management of elderly HNC patients. In a prospective study by Audisio et al., preoperative assessment of cancer in the elderly (PACE) was evaluated in 460 elderly patients with HNC cancer. Instrumental activities of daily living, abnormal performance status and moderate/severe fatigue were independent predictors of post-operative complications [70]. In a retrospective analysis of 100 patients aged 65 years and older, Serletti et al. reported that surgical time longer than 10 h is a predictive factor of postoperative surgical complications, whereas other factors include large fluid shifts and significant blood loss [71]. In addition, increased incidence of surgical and/ or medical complications (63% and 54%) was also reported in a retrospective analysis of 24 elderly HNC patients that underwent extensive surgical resection [62]. The use of minimally invasive techniques could be a feasible adaptation for elderly patients as this requires less operative and recovery time [72]. Another adaptation in an attempt to minimize the duration of surgery, could consist of omitting neck dissection in early stage HNC. However, in a French study of 270 patients with oral cavity cancer aged more than or equal to 80 years, neck dissections were omitted in 69% of patients. In these patients, neck node recurrence rate was significantly higher compared with patients who underwent neck dissection (40% vs. 6%) [17], showing clearly that these patients have been under-treated. Less morbid selective neck dissection procedures including selective neck dissection and sentinel lymph node biopsy are reasonable therapeutic adaptations which need to be further investigated. In a recent study in 22 patients with clinically T1/T2 oral squamous cell carcinoma of the tongue, 9.1% nodal recurrence was observed in patients who underwent sentinel node navigation surgery versus 27% recurrence in the control population, benefit proven no statistically significant [73]. For small, transorally accessible cancers of the oral cavity, pharynx, and larynx, surgical excision can be achieved with the use of microsurgical treatment, which incorporates the use of endoscopic laser or robotic techniques and high resolution magnified optics. This method allows functional preservation of much of the involved organ and good oncological results [74,75]. Transoral endoscopic surgery is well suited for elderly patients with cancer of the larynx. There are certain benefits that can be observed including limited hospital stay, less operative time, oncologically safe procedure in selected cases and satisfactory organ-function preservation [76]. More studies are required in order to produce more definite and applicable results. Controversy still surrounds microvascular free tissue transfer in elderly HNC population. Numerous retrospective studies show that reconstructive surgery with free flaps is feasible and safe option for most elderly HNC patients [68,77–79]. In a current study by Tarsitano et al. in 81 elderly (P75 years) and younger (<75 years) patients who underwent microsurgical free tissue transfer, the rates of major surgical complications were similar between both groups (11% vs. 9%) and no significant differences were observed concerning the rates of major and minor flap complications, morbidity or long-term functional outcome [79]. In the literature, the flap loss rate in different studies is variable and ranges from 1% out of 92 patients [80] to 16.7% out of 47 patients [65,81]. Blackwell et al. reported that free flap reconstruction in elderly HNC patients is feasible, but the incidence of medical complications and monetary cost have significantly increased [82]. The success rate of microvascular reconstruction is similar between older and younger HNC E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 patients, but medical and surgical post-operative complications seem to be directly related to the presence of comorbidities, rather than to age [78,83–86]. Conclusively, a careful selection of patients suitable for surgery in a multidimensional and multidisciplinary way is recommended. Several factors including the duration of operative time and the need for reconstruction with free tissue transfer, postoperative functional rehabilitation and postoperative irradiation, along with the patients’ wishes, should be considered before decision-making. A reduction in surgical stress, along with careful pre-operative staging, thorough assessment of associated comorbidities and skilful perioperative–postoperative management are essential for patients undergoing surgical resection in order to reduce postoperative morbidity and mortality. Radiotherapy Radiotherapy (RT) is an integral part of HNC therapy and can be delivered as definitive (radical RT), post-operative (adjuvant RT), or as palliative treatment. In HNC patients, RT allows a high rate of locoregional control with satisfactory organ-function preservation, and increased cure rates when delivered as single therapy for early stage glottic, base of tongue, and tonsillar cancer. The most widely used fractionation in patients with HNC is conventional fractionation of 1.8–2.0 Gy per fraction, delivered 5 days a week for more than 7 weeks for a total dose of 66–70 Gy for macroscopic disease and a total of 50–60 Gy for prophylactic treatment. In the literature, several studies exist to support that RT is generally effective and well tolerated in elderly HNC population, with similar outcomes compared with younger patients [21,30,87,88]. In a large retrospective study, 249 elders received radical RT and 59 elders received palliative RT for head and neck carcinomas. Treatment was well tolerated in both groups and no correlation between age and cancer-specific outcome was observed [21]. Similar results were reported in another retrospective study in 75 patients aged more than or equal to 75 years who were treated with curative intent for HNC. Survival rates were rapidly decreased within the first 2 years, but followed afterwards the survival rates of the general population [89]. Disease site and tumor stage were predictive factors of cancer-specific survival (59% at 5 years) and locoregional control (70% at 5 years) in a study of 98 patients aged between 80 and 92 years who received radical RT for HNC. In these patients, cancer-specific survival was similar to that of patients aged less than 80 years [87]. Increased age was not associated with increased risk of locoregional recurrence or reduced survival in another study in which 88 elderly patients received definitive RT and 16 patients palliative RT for oropharyngeal cancers, although older patients were treated with lower doses of radiation. No interaction between age and duration of therapy interruptions or severity of toxicity was observed [90]. In a retrospective review in 2312 patients, including 452 elderly (aged P75 years) and 1860 younger patients (aged <75 years) from a single institution, the 2-year cause-specific survival rate after definitive RT was significantly different between elderly and younger patients (72% vs. 86% p < 0.01), although no differences were found concerning treatment interruption, completion, or treatment-related death [5] Even in HNC patients aged more than 90 years, radiotherapy seems effective and well tolerated if it is individually planned as it was observed in a study by Oguchi et al. in 23 patients aged from 90 to 96 years. In this study, radiotherapy planning was based on disease stage and PS of the patients [91]. However, in a French study of 270 patients with oral cavity cancer aged more than or equal to 80 years, only 50% of the eligible, according to guidelines, patients underwent adjuvant radiotherapy after primary resection of the tumor and only 75% of patients were able to complete the treatment 469 [17]. Conclusively, current data suggest that age does not represent a limiting factor for radiotherapy, even in the ‘‘oldest old’’ subpopulation with good functional status, although a more personalized treatment planning and management are required. Advances in imaging and radiation techniques have improved treatment outcomes of radiotherapy while reducing functional deterioration and therapy-related morbidity. Improvements in radiation delivery offer the possibility of irradiating a limited target with increased doses while minimizing the unavoidable irradiated surrounding normal tissue. In addition, the frequent use of positron emission tomography/computed tomography (PET/CT) scan and magnetic resonance imaging (MRI) in treatment planning has allowed tumor delineation in three dimensions. Hyperfractionated external beam radiation therapy (HFRT) enables to deliver two to three fractions every day with a reduced dose per fraction, while maintaining or improving the toxicity profile of standard fractionation, despite an increased total dose. Accelerated fractionated radiotherapy aims at a reduction of overall treatment time by delivering more fractions during a short time and keeping the same total dose of irradiation as conventional RT. Both methods, commonly combined, represent an attractive method for elderly HNC patients. Several cooperative group (RTOG and EORTC) randomized trials have reported improved locoregional control and statistically nonsignificant survival benefit with hyperfractionated radiation therapy, while presenting slightly increased toxicity [92,93]. Between 1991 and 1997, 39 patients aged more than or equal to 70 years (mean age, 75 ± 6 years) presenting with carcinomas of the oral cavity, pharynx, or larynx were treated radically with accelerated RT. In all cases, the planned RT schedule was completed. Similar results with regard to 3-year actuarial overall survival and locoregional control were observed in both elderly and younger patients [88]. In a meta-analysis of 15 randomized trials, Bourhis et al. reported an advantage in overall survival and tumor control in patients receiving HFRT compared with conventional RT. However, lower compliance and decreased tolerance were observed in elderly patients with poor PS treated with HFRT [94]. In a more recent metanalysis, a significant survival benefit with altered fractionation radiotherapy was observed, along with a benefit in locoregional control. The benefit in overall survival was significantly higher with hyperfractionated radiotherapy (8% at 5 years) than with accelerated radiotherapy. However, stratification by age showed that the benefit was significantly higher in the youngest patients, while no benefit was observed in patients older than 70 years [95]. Intensity-modulated radiation therapy (IMRT) represents an advanced form of radiation delivery, in which the intensity is optimized to deliver a high dose of radiation to specified volumes, while reducing the dose and toxicity on adjacent non-target tissues. Thus, IMRT was developed to decrease the severity and likelihood of treatment-related morbidity, as critical organs and structures, including spinal cord, brain stem, brachial plexus, salivary glands, pharyngeal constrictors, and oral cavity, can be spared from high doses of radiation. In a phase III randomized trial (PASSPORT), parotid-sparing IMRT was compared to conventional RT in 90 patients with HNC. The incidence of xerostomia was significantly lower in the IMRT group at 12 and 24 months, while recovery of saliva secretion and improvements in associated QOL were observed with the use of IMRT [96]. However, in an analysis by Yu et al. in 1613 older patients (most aged P67 years) with HNC, the differences in 3-year overall survival (50.5% vs. 49.6% p = 0.47) and 3-year cancer-specific survival (60.0% vs. 58.8% p = 0.45) between IMRT and conventional RT, were no statistically significant [97]. Stereotactic body radiation therapy (SBRT) allows irradiating limited targets with high doses while protecting the surrounding normal tissue. There are studies reporting efficacy with limited 470 E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 toxicity in HNC patients [98]. It has been used for primary, recurrent and metastatic tumors in the head and neck region with excellent tolerance and is considered a promising ground for future research in elderly HNC patients [99] Proton therapy (PT) was reported to improve radiation dose distributions compared with Xray-based radiation therapy (RT) for selected cancers of the head and neck resulting in improved therapeutic ratio. Preliminary data in sinonasal tumors suggest significant improvements in disease control and radiation-related toxicity [100]. More studies in this field are needed in order to produce more definitive results for elderly HNC patients. Radiation-related toxicity is mainly caused by irradiation of the tumor-surrounding normal tissue. The most frequently observed acute side effects include mucositis, xerostomia (due to the irradiation of salivary glands), dysphagia, weight loss, pain, malnutrition, loss of taste, hoarseness and skin reactions, while other possible late complications include osteoradionecrosis, dental caries, subcutaneous fibrosis, trismus, thyroid dysfunction, sensorineural hearing loss, pharyngeal or oesophageal stenosis, and myelitis [8,59]. Data concerning radiation-related complications was reported by Pignon et al. regarding 1589 HNC patients who enrolled in EORTC trials. More than 20% of patients were aged more than or equal to 65 years and 2% aged more than or equal to 75 years. No significant difference in survival, acute mucosal reactions and weight loss more than 10% was observed between different age groups, although older patients had significantly more severe functional acute toxicity than younger patients. Regarding the late toxicity occurrence, no significant differences between ages were reported [101]. Thus, chronological age does not represent a key indicator for decision-making. In an attempt to minimize radiation-related toxicity, hypofractionated treatments were used for HNC patients. With the use of hypofractionation, increased doses of irradiation are delivered per fraction, while lowering the total number of fractions. In addition, these protocols can be delivered in a split-course manner with a 2- or 3-weeks interruption period during radiotherapy, thus limiting acute adverse events, such as malnutrition and functional decline [14,17,21]. In a French study of 270 patients aged more than or equal to 80 years, split-course hypofractionated radiotherapy was delivered in 56% of patients leading to good compliance and 81% rate of treatment completion [14]. However, further research is needed in order to examine the effectiveness and safety (given that increased doses per fraction may increase late toxicity) of this method in elderly HNC patients and produce more conclusive results. The prevalence of malnutrition in elderly HNC patients is high due to multiple causes including decreased taste and gastric secretion, dementia, depression, poor dentition, limited mobility, poverty and lack of caregiver. In addition, malnutrition is associated with a number of complications, including increased toxicity of surgery and radiotherapy [102–104], thus effective interventions in order to prevent or reverse malnutrition in older HNC patients are of great importance. The benefit of early nutritional intervention on QOL, treatment interruptions and risk of unplanned hospitalization has been assessed in several studies regarding HNC patients treated with radiotherapy [103,104]. Oral nutritional supplementation and feeding-tube placement could play a significant role in the management of these patients [105,106]. Summarizing, radiation therapy can be safely administered to elderly HNC population with both curative and palliative intent with high expectation of completion, although careful patient selection and attention to multiple parameters, including comorbidities, nutritional and functional status, are required in order to optimize outcome. Although older patients should be more closely monitored during radiotherapy, patients in good general condition should not be denied curative radiotherapy treatment, after being subjected to a thorough geriatric assessment; relevant treatment adaptations and individualized planning are recommended in order to minimize radiation-related toxicity, ensure good compliance, and limit the risk of post-radiation functional deterioration. Careful monitoring and patients’ management are always essential. Chemotherapy and chemoradiotherapy Chemotherapy has a multidimensional role in the management of HNC patients, either with palliative intent in metastatic and locally advanced incurable disease, or as a central component of curative programmes for locoregional HNC. Numerous agents such as platinum compounds, antimetabolites, and taxanes have shown activity against HNC as single agents, or in combination with radiotherapy or other agents. Chemotherapy in HNC can be delivered as induction (neoadjuvant), adjuvant or concomitant during a course of radiotherapy [8]. However, the role of chemotherapy in elderly HNC patients has not been well studied, due to the fact that older patients have generally been excluded from large randomized trials. In addition, a selection bias towards including healthier older patients in studies is also frequently observed. Moreover, due to changed renal function (reduced glomerular filtration rate) and changed hepatic metabolism (reduced activity of the microsomal oxidizing system), chemotherapy distribution is also changed in elderly patients resulting in pharmacokinetic and pharmacodynamic differences compared with younger patients [32,33]. Lastly, polypharmacy, which may lead to interactions with chemotherapeutic agents, should also be added in the aforementioned factors that could increase the risk of chemotherapy-related toxicity in the elderly population. In the induction setting, chemotherapy has the potential to reduce the risk of distant metastases, although the effect on locoregional control is inconclusive [8]. Cisplatin-based induction chemotherapy has produced response rates of 80–90%, with complete response rates of 20–40% in locally advanced HNC [107]. In a retrospective study in which patients up to 78 years of age were included, the combination of cisplatin/fluorouracil, as induction therapy, presented efficacy with response rates of 70–88% [108]. The randomized controlled trial RTOG 9-11, involving 547 Stage III or IV glottic or supraglottic squamous cell cancer patients and comparing induction chemotherapy (cisplatin/fluorouracil) followed by radiotherapy versus concomitant chemoradiotherapy (cisplatin) and radiotherapy alone, concluded that induction chemotherapy improved laryngectomy-free survival versus radiotherapy alone (p = 0.02) and so did concomitant chemoradiotherapy (p = 0.03) [109]. However, it did not improve overall survival or larynx preservation rate over radiotherapy alone. The introduction of taxane-containing chemotherapy combinations has increased the efficacy and strengthened the role of neoadjuvant chemotherapy in the management of HNC patients [8]. The combination of a taxane (docetaxel or paclitaxel) plus cisplatin and fluorouracil in phase III clinical trials, has resulted in improved survival and organ preservation compared with cisplatin and fluorouracil alone [110– 113]. In one study, in which the addition of docetaxel in the cisplatin/fluorouracil combination, as neoadjuvant treatment, resulted in a 12% survival benefit, HNC patients up to 82 years old were enrolled [112]. In another study, in which the combination of docetaxel/cisplatin/fluorouracil resulted in a significant improve in progression free and overall survival compared with the standard cisplatin/fluorouracil regimen, 10% of the patients participating in the study were between 65 and 71 years old [113]. However, induction chemotherapy followed by chemoradiotherapy has been found to be associated with a high rate of rate of severe acute and late RT-related toxicities, which must be taken in special E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 consideration in the elderly patients, with their comorbidities and worse functional status [114]. A major advancement in the treatment of HNC patients with locally advanced disease has been the concurrent administration of chemotherapy and radiotherapy, known as chemoradiotherapy. Numerous phase III studies have reported better results with the use of chemoradiotherapy than radiotherapy alone or sequential administration of chemotherapy and radiotherapy [8], although chemoradiation has been associated with significant acute complications, including mucositis, dysphagia, and skin reactions; toxicities that affect patients’ compliance and often interfere with treatment delivery [115,116]. Late toxicities such as pharyngoesophageal stenosis and dysfunction resulting in feeding tube dependence, cervical fibrosis, tracheal stenosis and osteoradionecrosis are also observed [117–119], and have been found to be more frequently reported in older patients [120,121]. In a phase I study of 15 head and neck cancer patients aged more than or equal to 70 years, the addition of docetaxel to irradiation was well tolerated even though grade 3–4 mucositis was still significant [122]. Benefit from chemoradiotherapy has also been documented in the postoperative setting. Non age-specific phase III studies reported that the addition of cisplatin-based chemotherapy to postsurgical radiotherapy improved locoregional control, disease-free survival, or overall survival [8], although the benefit was greater when extracapsular spread or positive margins were present [123]. The Meta-Analysis of Chemotherapy in Head and Neck Cancer (MACH-NC), the largest head and neck cancer meta-analysis reported, concluded that the survival benefit from adding chemotherapy to locoregional treatment actually decreased with increasing age and that there was no benefit at all in patients older than 70 years [16]. In contrast, in a single institution retrospective study in which 44 HNC patients aged more than or equal to 70 years (range, 70–77; median, 72) and 137 patients aged less than 70 years (range, 24–69; median, 56) were enrolled and received cisplatin-based concurrent chemoradiotherapy, projected 5-year disease-specific survival (71% vs. 74%) and freedom from recurrence (69% vs. 71%) were nearly identical between older and younger patients. Clinical characteristics, treatment and toxicities were similar except that the elderly were less likely to complete chemotherapy courses, experienced more myelosuppression, required more frequent unplanned hospitalization, and were longer feeding-tube dependent [124]. However, a selection bias towards including healthier older patients to receive chemoradiotherapy, along with an increased heterogeneity of patients’ population were once again present in this study. A single center retrospective study comparing the tolerance of elderly and younger patients to concurrent chemotherapy and radiotherapy using modern radiotherapy techniques was conducted by Nguyen et al. enrolling 112 patients with locally advanced HNC. Eighty-five patients were less than 70 years old (median age 60 years) and 27 patients were more or equal to 70 years old (median age 74 years), while all patients were treated with intensity-modulated radiotherapy (IMRT) or image-guided radiotherapy (IGRT) technique and concurrent chemotherapy. The 2-year survival was estimated to be 67.5% for the elderly patients and 74% for younger patients, respectively, and there was no difference in grade 3–4 toxicity or treatment interruptions between the two age groups. Authors reported that, even though the patients’ number was small with a short follow-up, elderly patients seemed to tolerate the combined modality as well as younger patients. However, acute toxicity was still significant in both groups of patients and that all patients required meticulous attention to radiotherapy techniques and strong nutritional support during treatment [125]. In patients with recurrent or metastatic HNC several agents including methotrexate, bleomycin, carboplatin, and fluorouracil 471 have shown activity as single agents [126]. In randomized trials, two-drug combinations improved response rates but not overall survival [126]. Cisplatin plus fluorouracil has been widely accepted as a reference regimen in patients with recurrent or metastatic HNC achieving a response rate of 40–50%, although a platinum/taxane combination is also an acceptable alternative [127]. Data from two randomized phase III trials conducted by the Eastern Cooperative Oncology Group (ECOG protocol E1393 and E1395) comparing the toxicity, response rates, and survival of HNC patients aged more than or equal to 70 years versus their younger counterparts, were reported by Argiris et al. Elderly patients were underrepresented in these studies, thus only 53 older patients were eventually enrolled. Similar objective response rates (28% vs. 33%) and median time to progression (5.25 vs. 4.8 months) along with no statistically significant differences in median survival (5.3 vs. 8 months) and 1-year survival (26% vs. 33%) between elderly and younger patients were reported. A significantly higher incidence of toxicity, including severe nephrotoxicity, diarrhea, and thrombocytopenia and a higher statistically nonsignificant rate of toxic deaths were reported in elderly patients. Authors concluded that although survival outcomes between older and younger patients were comparable, elderly patients sustained increased toxicities compared with younger counterparts [31]. Despite the increase in toxicity, it is generally recommended that older patients with a good PS should receive the same standard of care treatment as younger patients [128], because reduction in chemotherapy dose was found to seriously mitigate the efficacy of treatment [129]. Thus, effective management of the chemotherapyassociated toxicity with appropriate supportive care is crucial in order to provide elderly HNC population a chance for safe and effective treatment [130]. In conclusion, age per se should not be considered a contraindication to aggressive multiagent chemotherapy and concurrent chemoradiotherapy treatment, although the efficacy of the later in elderly HNC patients is still under investigation. However, given that elders are more prone to develop chemotherapy-induced toxicity, there is a critical need to define the criteria that will predict patient’s ability to tolerate aggressive chemotherapy and, more importantly, to identify less toxic but equally effective treatment regimens. Lastly, there is an imperative need for prospective older-specific trials that will produce results more focused on older population, improve accrual and withdraw reluctance on the part of treating oncologists to enroll older patients in clinical trials (Fig. 2). Novel agents In an attempt to identify less toxic but equally effective treatment regimens, epidermal growth factor receptor (EGFR) inhibition has emerged as a novel treatment strategy for HNC [131]. Monoclonal antibodies, small-molecule tyrosine-kinase inhibitors targeting EGFR and other dysregulated molecular pathways, along with vascular endothelial growth factor receptor (VEGFR) inhibitors and proteasome inhibitors are still under investigation in HNC, whereas the combination of these novel agents with chemotherapy and radiotherapy is currently being explored. Cetuximab, an IgG1 chimeric monoclonal antibody highly selective for the EGFR, is the first molecularly targeted agent that has been introduced into clinical practice for HNC [131]. In patients with locally advanced HNC, the combination of radiation and cetuximab (concomitant weekly cetuximab 250 mg/m2 after an initial 400 mg/m2 loading dose) presented a significant benefit in locoregional control (47% vs. 34% at 3 years p = 0.005), progression-free survival (42% vs. 31% at 3 years p = 0.006), and overall survival (55% vs. 45% at 3 years p = 0.03) compared with radiation 472 E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 Fig. 2. Factors favoring and factors against older-specific trials. alone. No differences concerning distant control, radiation-related toxicity or QOL were reported [132]. The combination of radiation with cetuximab is an alternative to chemoradiotherapy that should be strongly considered in patients who cannot tolerate chemotherapy, since this regimen is not associated with increased myelosuppression or mucositis, although complications due to inhibition of the EGFR pathway are reported, including rash and hypomagnesaemia. However, in a recent update of this trial analyzing the impact of age, authors reported no benefit for concurrent cetuximab in patients aged more than 65 years [133], although the lack of statistical power in this study due to the low number of patients aged more than 65 years should be highlighted. In contrast, a retrospective single-center study of concurrent cetuximab plus radiation was conducted by Jensen et al. in elderly and multi-morbid patients with primary or recurrent HNC. Seventy-three patients with a median age of 69 years (range from 42 to 86 years) were enrolled in this study and received radioimmunotherapy (RIT) with cetuximab instead of chemoradiation due to poor overall PS and multiple co-morbidities. Twenty-two of the patients participating in the study received RIT for re-irradiation. Overall response rate was 59.4%, median locoregional and overall progression-free survival was 18 and 15 months respectively, and an overall survival of 18 months was also reported. In 4 patients grade 3 allergic reactions at first exposure occurred, whereas grade 3 skin reactions leading to discontinuation of cetuximab were reported in 3 patients. Authors concluded that concurrent cetuximab plus radiation is feasible in elderly and multi-morbid patients with promising therapeutic activity, while local control can still be achieved in patients treated for local relapse of their disease or undergoing re-irradiation for recurrent HNC [134]. The feasibility and acute toxicity of cetuximab plus volumetric modulation arc therapy (VMAT) in elderly and chemotherapy-ineligible patients was investigated by Alongi et al. in 22 patients with locally advanced HNC. None of the patients enrolled in the study was suitable for chemotherapy because of important comorbidities. The most frequently observed side effects were dermatitis and mucositis/stomatitis, while no grade 4 toxicities were recorded. Authors concluded that the combination of cetuximab plus VMAT seems feasible in elderly and multi-morbid HNC patients [135]. However, a direct comparison of radiation and conventional chemotherapy with radiation and cetuximab has not yet been performed in elderly HNC patients in order to have more definitive results. For those patients who cannot tolerate high-dose cisplatin therapy concurrently with radiation therapy, cetuximab presents as a potential option which, however, should be further investigated (Table 1). The role of cetuximab in patients with recurrent or metastatic HNC was investigated in a randomized trial, in which 123 patients received cisplatin with or without cetuximab as first-line treatment. Researchers reported that the addition of cetuximab to cisplatin significantly improves response rate [136]. In a phase III study in 420 HNC patients, the combination of a platinum agent and fluorouracil with or without cetuximab was investigated. The addition of cetuximab resulted in an overall survival benefit (10.1 vs. 7.4 months p = 0.036), but the beneficial effects of adding cetuximab disappeared in the subgroup of elderly (P65 years) patients (hazard ratio, 1.07; 95% CI, 0.65–1.77) [137]. Taking into consideration that elderly patients have been generally underrepresented in these studies and that no older-specific trials exist to support the efficacy and safety of cetuximab in elderly HNC patients with recurrent or metastatic disease, further research is required before this agent is introduced in every-day clinical practice for this specific subpopulation. EGFR-tyrosine kinase inhibitors, gefitinib or erlotinib, have also been explored in patients with recurrent or metastatic HNC [131]. In a phase III study enrolling 486 patients with recurrent HNC, gefitinib 250 or 500 mg/day failed to present benefit against intravenous methotrexate in terms of overall survival or overall response rates [138]. A recently published randomized, placebo-controlled phase III trial investigated the therapeutic efficacy of adding gefitinib to docetaxel in patients with recurrent or metastatic HNC. Two hundred seventy patients were enrolled and although the addition of gefitinib to docetaxel was well tolerated, no statistically significant difference in overall survival was reported (6.0 vs. 7.3 months Table 1 Ongoing trials with cetuximab in elderly HNC patients (source: www.clinicaltrials.gov). Title Phase Protocol ID Eligible patients Treatment Mechanism Study of radiation (RT) concurrent with cetuximab in patients with advanced head and neck squamous cell carcinoma (SCC) Phase 2 NCT00904345 Patients with locally advanced head and neck SCC who do not qualify for standard chemotherapy due to age > 70 or co-morbidities Radiation concurrent with cetuximab Anti-EGFR Radiation with cetuximab in head and neck squamous cell carcinoma (SCC) who do not qualify for chemotherapy Phase 2 NCT01250522 Patients with locally advanced head and neck SCC who do not qualify for standard chemotherapy due to age > 70 or co-morbidities Radiation concurrent with cetuximab Anti-EGFR Safety and efficacy of radiation/cetuximab plus EGFR antisense DNA for head and neck squamous cell carcinoma Phase 1 + phase 2 NCT00903461 Elderly or cisplatin-ineligible patients with locally advanced head and neck SCC Radiation/ cetuximab plus EGFR antisense DNA Anti-EGFR E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 p = 0.60). A subset analysis, however, showed that gefitinib managed to improve survival only in patients younger than 65 years, while patients older than 65 years failed to present any benefit [139]. Panitumumab is a fully human monoclonal antibody directed against EGFR that is currently being investigated in HNC. In a phase III study in 657 patients with recurrent or metastatic HNC aged up to 84 years, the use of panitutmumab in combination with conventional chemotherapy did not present any benefit in terms of overall survival compared with chemotherapy alone [140]. However, a later subgroup analysis showed that HPV-negative patients had improved overall survival and progression free survival when panitumumab was added to conventional chemotherapy, whereas no improvements were observed in patients with HPV-positive tumors [141]. Taking into consideration that HPV infection seems to have minor effect in the pathogenesis of HNC in the elderly population and that the majority of older HNC patients have HPV-negative tumors [20], panitumumab seems as a promising agent for further research in the geriatric HNC population. Several more targeted agents, including monoclonal antibodies, single-selective or multi-selective tyrosine kinase inhibitors, and nucleic acid-directed approaches are currently under research in HNC patients. The potential efficacy of these novel agents is of great importance for the elderly HNC patients in an attempt to reduce and minimize the severe chemotherapy-induced toxicity which is very commonly observed in this specific subpopulation. Conclusion Treatment selection for elderly HNC patients is surrounded by a high rate of controversy and remains a challenging issue for oncologists. Practice-changing large randomized trials have generally excluded older patients, resulting in a lack of evidence-based data with regard to the most appropriate treatment for this specific subgroup. Decisions for diagnosis and treatment are often complex and have to be evaluated in the frame of a multidisciplinary approach including the participation of many specialists and a thorough oncogeriatric assessment. Advanced age per se should not be considered a contraindication to curative treatment including surgery, radiotherapy and/or chemotherapy. Other factors, including comorbidities, disabilities, frailty, and impaired functional status are considered to be more relevant criteria than chronological age by itself for decision making. Thus, a comprehensive geriatric assessment, along with a multidisciplinary approach and treatment adaptations that will minimize therapy-related toxicity, are considered essential. The primary treatment goal should be firmly determined (cure or relieve) and the best-tailored individualized therapeutic option should be selected in order to avoid high toxicity and post-treatment significant functional decline. Advances in surgical and radiation techniques, have improved treatment outcomes for elderly HNC patients while reducing functional deterioration and therapy-related morbidity. In addition, improvements in chemotherapy delivery, along with the induction of new molecularly targeted agents in clinical practice, have managed to reduce the severe chemotherapy-related complications. However, the necessity for prospective older-specific trials that will produce results more focused on older population and withdraw reluctance on the part of treating oncologists to enroll older patients in clinical trials, should be highlighted. New treatment approaches for the elderly HNC population are currently being investigated. Conflict of interest statement The authors declare no conflict of interest. 473 References [1] Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108. [2] Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin 2007;57:43–66. [3] Syrigos KN, Karachalios D, Karapanagiotou EM, Nutting CM, Manolopoulos L, Harrington KJ. Head and neck cancer in the elderly: an overview on the treatment modalities. Cancer Treat Rev 2009;35:237–45. [4] Kruse AL, Bredell M, Luebbers HT, Gratz KW. Head and neck cancer in the elderly: a retrospective study over 10 years. Head Neck Oncol 2010;2:25. [5] Huang SH, O’Sullivan B, Waldron J, et al. Patterns of care in elderly head-andneck cancer radiation oncology patients: a single-center cohort study. Int J Radiat Oncol Biol Phys 2011;79:46–51. [6] Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 2009;27:2758–65. [7] Finnish Cancer Registry. Available from: <http://www.cancerregistry.fi>. [8] Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet 2008;371:1695–709. [9] Argiris A, Eng C. Epidemiology, staging, and screening of head and neck cancer. Cancer Treat Res 2003;114:15–60. [10] Pallis AG, Fortpied C, Wedding U, et al. EORTC elderly task force position paper: approach to the older cancer patient. Eur J Cancer 2010;46:1502–13. [11] Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin 1997;47:5–27. [12] Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol 2003;21(7):1383–9. [13] Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol 2004;22(22):4626–31. [14] Italiano A, Ortholan C, Dassonville O, et al. Head and neck squamous cell carcinoma in patients aged > or = 80 years: patterns of care and survival. Cancer 2008;113:3160–8. [15] Sarini J, Fournier C, Lefebvre JL, Bonafos G, Van JT, Coche-Dequeant B. Head and neck squamous cell carcinoma in elderly patients: a long-term retrospective review of 273 cases. Arch Otolaryngol Head Neck Surg 2001;127:1089–92. [16] Pignon JP, le Maître A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92:4–14. [17] Ortholan C, Lusinchi A, Italiano A, et al. Oral cavity squamous cell carcinoma in 260 patients aged 80years or more. Radiother Oncol 2009;93:516–23. [18] Koch WM, Patel H, Brennan J, Boyle JO, Sidransky D. Squamous cell carcinoma of the head and neck in the elderly. Arch Otolaryngol Head Neck Surg 1995;121:262–5. [19] Leon X, Quer M, Agudelo D, et al. Influence of age on laryngeal carcinoma. Ann Otol Rhinol Laryngol 1998;107:164–9. [20] Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [21] Boscolo-Rizzo P, Del Mistro A, Bussu F, Lupato V, Baboci L, Almadori G, et al. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital 2013;33(2):77–87. [22] Dayyani F, Etzel CJ, Liu M, Ho CH, Lippman SM, Tsao AS. Meta-analysis of the impact of human papillomavirus (HPV) on cancer risk and overall survival in head and neck squamous cell carcinomas (HNSCC). Head Neck Oncol 2010;29(2):15. [23] Ragin CC, Taioli E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: review and meta-analysis. Int J Cancer 2007;121(8):1813–20. [24] Lusinchi A, Bourhis J, Wibault P, Le Ridant AM, Eschwege F. Radiation therapy for head and neck cancers in the elderly. Int J Radiat Oncol Biol Phys 1990;18:819–23. [25] Jones AS, Husband D, Rowley H. Radical radiotherapy for squamous cell carcinoma of the larynx, oropharynx and hypopharynx: patterns of recurrence, treatment and survival. Clin Otolaryngol Allied Sci 1998;23:496–511. [26] Hirano M, Mori K. Management of cancer in the elderly: therapeutic dilemmas. Otolaryngol Head Neck Surg 1998;118:110–4. [27] Derks W, de Leeuw JR, Hordijk GJ, Winnubst JA. Reasons for non-standard treatment in elderly patients with advanced head and neck cancer. Eur Arch Otorhinolaryngol 2005;262:21–6. [28] Bernardi D, Barzan L, Franchin G, et al. Treatment of head and neck cancer in elderly patients: state of the art and guidelines. Crit Rev Oncol Hematol 2005;53:71–80. [29] Clayman GL, Eicher SA, Sicard MW, Razmpa E, Goepfert H. Surgical outcomes in head and neck cancer patients 80 years of age and older. Head Neck 1998;20:216–23. [30] Airoldi M, Cortesina G, Giordano C, et al. Postoperative adjuvant chemoradiotherapy in older patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2004;130:161–6. [31] Argiris A, Li Y, Murphy BA, Langer CJ, Forastiere AA. Outcome of elderly patients with recurrent or metastatic head and neck cancer treated with cisplatin-based chemotherapy. J Clin Oncol 2004;22:262–8. 474 E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 [32] Bhattacharyya N. A matched survival analysis for squamous cell carcinoma of the head and neck in the elderly. Laryngoscope 2003;113:368–72. [33] Extermann M, Aapro M, Bernabei R, et al. Use of comprehensive geriatric assessment in older cancer patients: recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit Rev Oncol Hematol 2005;55:241–52. [34] Balducci L, Beghe’ C. Cancer and age in the USA. Crit Rev Oncol Hematol 2001;37:137–45. [35] Syrigos KN, Karapanagiotou E, Charpidou A, et al. Biweekly administration of docetaxel and gemcitabine for elderly patients with advanced non-small cell lung cancer: a phase II study. J Chemother 2007;19:438–43. [36] Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol 1998;16:1582–7. [37] Priebe HJ. The aged cardiovascular risk patient. Br J Anaesth 2000;85:763–78. [38] Lakatta EG. Cardiovascular aging research: the next horizons. J Am Geriatr Soc 1999;47:613–25. [39] Paleri V, Wight RG, Silver CE, et al. Comorbidity in head and neck cancer: a critical appraisal and recommendations for practice. Oral Oncol 2010;46:712–9. [40] Bond SM, Dietrich MS, Murphy BA. Association of age and symptom burden in patients with head and neck cancer. ORL Head Neck Nurs 2011;29:8–14. [41] Murphy BA, Ridner S, Wells N, Dietrich M. Quality of life research in head and neck cancer: a review of the current state of the science. Crit Rev Oncol Hematol 2007;62:251–67. [42] Sanabria A, Carvalho AL, Vartanian JG, Magrin J, Ikeda MK, Kowalski LP. Factors that influence treatment decision in older patients with resectable head and neck cancer. Laryngoscope 2007;117:835–40. [43] El-Deiry MW, Futran ND, McDowell JA, Weymuller Jr EA, Yueh B. Influences and predictors of long-term quality of life in head and neck cancer survivors. Arch Otolaryngol Head Neck Surg 2009;135:380–4. [44] Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg 2004;130:401–8. [45] Gourin CG, Boyce BJ, Vaught CC, Burkhead LM, Podolsky RH. Effect of comorbidity on post-treatment quality of life scores in patients with head and neck squamous cell carcinoma. Laryngoscope 2009;119:907–14. [46] Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel Jr EL. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA 2004;291:2441–7. [47] Wan LS, Lee TF, Chien CY, Chao PJ, Tsai WL, Fang FM. Health-related quality of life in 640 head and neck cancer survivors after radiotherapy using EORTC QLQ-C30 and QLQ-H&N35 questionnaires. BMC Cancer 2011;11:128. [48] Fang FM, Liu YT, Tang Y, Wang CJ, Ko SF. Quality of life as a survival predictor for patients with advanced head and neck carcinoma treated with radiotherapy. Cancer 2004;100:425–32. [49] Gourin CG, McAfee WJ, Neyman KM, Howington JW, Podolsky RH, Terris DJ. Effect of comorbidity on quality of life and treatment selection in patients with squamous cell carcinoma of the head and neck. Laryngoscope 2005;115:1371–5. [50] Peters TT, van der Laan BF, Plaat BE, Wedman J, Langendijk JA, Halmos GB. The impact of comorbidity on treatment-related side effects in older patients with laryngeal cancer. Oral Oncol 2011;47:56–61. [51] Borggreven PA, Verdonck-de Leeuw IM, Muller MJ, et al. Quality of life and functional status in patients with cancer of the oral cavity and oropharynx: pretreatment values of a prospective study. Eur Arch Otorhinolaryngol 2007;264:651–7. [52] Oozeer NB, Benbow J, Downs C, Kelly C, Welch A, Paleri V. The effect of comorbidity on quality of life during radiotherapy in head and neck cancer. Otolaryngol Head Neck Surg 2008;139:268–72. [53] Sanabria A, Carvalho AL, Vartanian JG, Magrin J, Ikeda MK, Kowalski LP. Comorbidity is a prognostic factor in elderly patients with head and neck cancer. Ann Surg Oncol 2007;14:1449–57. [54] Bernabei R, Venturiero V, Tarsitani P, Gambassi G. The comprehensive geriatric assessment: when, where, how. Crit Rev Oncol Hematol 2000;33:45–56. [55] Metges JP, Eschwege F, de Crevoisier R, Lusinchi A, Bourhis J, Wibault P. Radiotherapy in head and neck cancer in the elderly: a challenge. Crit Rev Oncol Hematol 2000;34:195–203. [56] Lalami Y, de Crevoisier Jr G, Bernard-Marty C, Awada A. Management of head and neck cancer in elderly patients. Drugs Aging 2009;26:571–83. [57] Luciani A, Ascione G, Bertuzzi C, et al. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol 2010;28:2046–50. [58] Balducci L, Extermann M. Management of cancer in the older person: a practical approach. Oncologist 2000;5:224–37. [59] Ortholan C, Benezery K, Dassonville O, et al. A specific approach for elderly patients with head and neck cancer. Anticancer Drugs 2011;22:647–55. [60] Morgan RF, Hirata RM, Jaques DA, Hoopes JE. Head and neck surgery in the aged. Am J Surg 1982;144:449–51. [61] McGuirt WF, Loevy S, McCabe BF, Krause CJ. The risks of major head and neck surgery in the aged population. Laryngoscope 1977;87:1378–82. [62] Zabrodsky M, Calabrese L, Tosoni A, et al. Major surgery in elderly head and neck cancer patients: immediate and long-term surgical results and complication rates. Surg Oncol 2004;13:249–55. [63] Laccourreye O, Brasnu D, Perie S, Muscatello L, Menard M, Weinstein G. Supracricoid partial laryngectomies in the elderly: mortality, complications, and functional outcome. Laryngoscope 1998;108:237–42. [64] Teymoortash A, Wulf H, Werner JA. Head and neck cancer surgery in the elderly. Laryngorhinootologie 2002;81:293–8. [65] Jones AS, Beasley N, Houghton D, Husband DJ. The effects of age on survival and other parameters in squamous cell carcinoma of the oral cavity, pharynx and larynx. Clin Otolaryngol Allied Sci 1998;23:51–6. [66] Boruk M, Chernobilsky B, Rosenfeld RM, Har-El G. Age as a prognostic factor for complications of major head and neck surgery. Arch Otolaryngol Head Neck Surg 2005;131:605–9. [67] Ferrier MB, Spuesens EB, Le CS, Baatenburg de Jong RJ. Comorbidity as a major risk factor for mortality and complications in head and neck surgery. Arch Otolaryngol Head Neck Surg 2005;131:27–32. [68] Derks W, De Leeuw JR, Hordijk GJ, Winnubst JA. Elderly patients with head and neck cancer: short-term effects of surgical treatment on quality of life. Clin Otolaryngol Allied Sci 2003;28:399–405. [69] Sanabria A, Carvalho AL, Melo RL, et al. Predictive factors for complications in elderly patients who underwent head and neck oncologic surgery. Head Neck 2008;30:170–7. [70] Audisio RA, Pope D, Ramesh HS, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63. [71] Serletti JM, Higgins JP, Moran S, Orlando GS. Factors affecting outcome in free-tissue transfer in the elderly. Plast Reconstr Surg 2000;106:66–70. [72] Werner JA, Dunne AA, Folz BJ, Lippert BM. Transoral laser microsurgery in carcinomas of the oral cavity, pharynx, and larynx. Cancer Control 2002;9:379–86. [73] Yamauchi K, Fujioka Y, Kohno N. Sentinel node navigation surgery versus observation as a management strategy for early tongue carcinoma. Head Neck 2012;34:568–72. [74] Ambrosch P. The role of laser microsurgery in the treatment of laryngeal cancer. Curr Opin Otolaryngol Head Neck Surg 2007;15:82–8. [75] Lefebvre JL. Laryngeal preservation in head and neck cancer: multidisciplinary approach. Lancet Oncol 2006;7:747–55. [76] Grenman R, Chevalier D, Gregoire V, Myers E, Rogers S. Treatment of head and neck cancer in the elderly: European Consensus (panel 6) at the EUFOS Congress in Vienna 2007. Eur Arch Otorhinolaryngol 2010;267:1619–21. [77] Chick LR, Walton RL, Reus W, Colen L, Sasmor M. Free flaps in the elderly. Plast Reconstr Surg 1992;90:87–94. [78] Malata CM, Cooter RD, Batchelor AG, Simpson KH, Browning FS, Kay SP. Microvascular free-tissue transfers in elderly patients: the leeds experience. Plast Reconstr Surg 1996;98:1234–41. [79] Tarsitano A, Pizzigallo A, Sgarzani R, Oranges CM, Cipriani R, Marchetti C. Head and neck cancer in elderly patients: is microsurgical free-tissue transfer a safe procedure? Acta Otorhinolaryngol Ital 2012;32:371–5. [80] Shestak KC, Jones NF. Microsurgical free-tissue transfer in the elderly patient. Plast Reconstr Surg 1991;88:259–63. [81] Bonawitz SC, Schnarrs RH, Rosenthal AI, Rogers GK, Newton ED. Free-tissue transfer in elderly patients. Plast Reconstr Surg 1991;87:1074–9. [82] Blackwell KE, Azizzadeh B, Ayala C, Rawnsley JD. Octogenarian free flap reconstruction: complications and cost of therapy. Otolaryngol Head Neck Surg 2002;126:301–6. [83] Shestak KC, Jones NF, Wu W, Johnson JT, Myers EN. Effect of advanced age and medical disease on the outcome of microvascular reconstruction for head and neck defects. Head Neck 1992;14:14–8. [84] Bridger AG, O’Brien CJ, Lee KK. Advanced patient age should not preclude the use of free-flap reconstruction for head and neck cancer. Am J Surg 1994;168:425–8. [85] Shaari CM, Buchbinder D, Costantino PD, Lawson W, Biller HF, Urken ML. Complications of microvascular head and neck surgery in the elderly. Arch Otolaryngol Head Neck Surg 1998;124:407–11. [86] Pompei S, Tedesco M, Pozzi M, Varanese A, Barile A, Marzetti F. Age as a risk factor in cervicofacial reconstruction. J Exp Clin Cancer Res 1999;18: 209–12. [87] Schofield CP, Sykes AJ, Slevin NJ, Rashid NZ. Radiotherapy for head and neck cancer in elderly patients. Radiother Oncol 2003;69:37–42. [88] Allal AS, Maire D, Becker M, Dulguerov P. Feasibility and early results of accelerated radiotherapy for head and neck carcinoma in the elderly. Cancer 2000;88:648–52. [89] Huguenin P, Sauer M, Glanzmann C, Lutolf UM. Radiotherapy for carcinomas of the head and neck in elderly patients. Strahlenther Onkol 1996;172:485–8. [90] Schilcher B, Curschmann J. Clinical results of radiotherapy in 140 elderly patients treated at Basel University Hospital between 1980 and 1985. Int J Radiat Oncol Biol Phys 1995;33:774. [91] Oguchi M, Ikeda H, Watanabe T, et al. Experiences of 23 patients > or = 90 years of age treated with radiation therapy. Int J Radiat Oncol Biol Phys 1998;41:407–13. [92] Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys 2000;48:7–16. [93] Horiot JC, Le FR, N’Guyen T, et al. Hyperfractionated compared with conventional radiotherapy in oropharyngeal carcinoma: an EORTC randomized trial. Eur J Cancer 1990;26:779–80. E.G. Sarris et al. / Cancer Treatment Reviews 40 (2014) 465–475 [94] Bourhis J, Overgaard J, Audry H, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet 2006;368:843–54. [95] Baujat B, Bourhis J, Blanchard P, et al. Hyperfractionated or accelerated radiotherapy for head and neck cancer. Cochrane Database Syst Rev 2010:CD002026. [96] Nutting CM, Morden JP, Harrington KJ, et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol 2011;12:127–36. [97] Yu JB, Soulos PR, Sharma R, et al. Patterns of care and outcomes associated with intensity-modulated radiation therapy versus conventional radiation therapy for older patients with head-and-neck cancer. Int J Radiat Oncol Biol Phys 2012;83:e101–7. [98] Siddiqui F, Raben D, Lu JJ, et al. Emerging applications of stereotactic body radiation therapy for head and neck cancer. Expert Rev Anticancer Ther 2011;11:1429–36. [99] Siddiqui F, Patel M, Khan M, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys 2009;74:1047–53. [100] Mendenhall NP, Malyapa RS, Su Z, Yeung D, Mendenhall WM, Li Z. Proton therapy for head and neck cancer: rationale, potential indications, practical considerations, and current clinical evidence. Acta Oncol 2011;50: 763–71. [101] Pignon T, Horiot JC, Van den Bogaert W, Van GM, Scalliet P. No age limit for radical radiotherapy in head and neck tumours. Eur J Cancer 1996;32A:2075–81. [102] Guo CB, Ma DQ, Zhang KH, Hu XH. Relation between nutritional state and postoperative complications in patients with oral and maxillofacial malignancy. Br J Oral Maxillofac Surg 2007;45:467–70. [103] Ravasco P, Monteiro-Grillo I, Marques VP, Camilo ME. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck 2005;27:659–68. [104] Paccagnella A, Morello M, Da Mosto MC, et al. Early nutritional intervention improves treatment tolerance and outcomes in head and neck cancer patients undergoing concurrent chemoradiotherapy. Support Care Cancer 2010;18:837–45. [105] Chen AM, Li BQ, Lau DH, et al. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2010;78:1026–32. [106] Lee H, Havrila C, Bravo V, et al. Effect of oral nutritional supplementation on weight loss and percutaneous endoscopic gastrostomy tube rates in patients treated with radiotherapy for oropharyngeal carcinoma. Support Care Cancer 2008;16:285–9. [107] Argiris A. Induction chemotherapy for head and neck cancer: will history repeat itself? J Natl Compr Canc Netw 2005;3:393–403. [108] Bhide SA, Ahmed M, Barbachano Y, Newbold K, Harrington KJ, Nutting CM. Sequential induction chemotherapy followed by radical chemo-radiation in the treatment of locoregionally advanced head-and-neck cancer. Br J Cancer 2008;99:57–62. [109] Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, et al. Longterm results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31(7):845–52. [110] Calais G, Pointreau Y, Alfonsi M, et al. Randomized phase III trial comparing induction chemotherapy using cisplatin (P) fluorouracil (F) with or without docetaxel (T) for organ preservation in hypopharynx and larynx cancer. Preliminary results of GORTEC 2000-01. J Clin Oncol 2006;24(Suppl. 18):5506. [111] Hitt R, Lopez-Pousa A, Martinez-Trufero J, et al. Phase III study comparing cisplatin plus fluorouracil to paclitaxel, cisplatin, and fluorouracil induction chemotherapy followed by chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol 2005;23:8636–45. [112] Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 2007;357:1705–15. [113] Vermorken JB, Remenar E, Van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 2007;357:1695–704. [114] Brömme JO, Schmücking M, Arnold A, Giger R, Rauch D, Leiser D, et al. Taxane-containing induction chemotherapy followed by definitive chemoradiotherapy. Outcome in patients with locally advanced head and neck cancer. Strahlenther Onkol 2013;189(8):618–24. [115] Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol 2003;66:253–62. [116] Rosenthal DI, Lewin JS, Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol 2006;24:2636–43. [117] Trotti A. Toxicity in head and neck cancer: a review of trends and issues. Int J Radiat Oncol Biol Phys 2000;47:1–12. 475 [118] Bentzen SM, Rosenthal DI, Weymuller EA, Trotti A. Increasing toxicity in nonoperative head and neck cancer treatment: investigations and interventions. Int J Radiat Oncol Biol Phys 2007;69:S79–82. [119] Bentzen SM, Trotti A. Evaluation of early and late toxicities in chemoradiation trials. J Clin Oncol 2007;25:4096–103. [120] Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol 2008;26:3582–9. [121] Caudell JJ, Schaner PE, Meredith RF, et al. Factors associated with long-term dysphagia after definitive radiotherapy for locally advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys 2009;73:410–5. [122] Kodaira T, Fuwa N, Furutani K, Tachibana H, Yamazaki T. Phase I trial of weekly docetaxel and concurrent radiotherapy for head and neck cancer in elderly patients or patients with complications. Jpn J Clin Oncol 2005;35:173–6. [123] Bernier J, Cooper JS, Pajak TF, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck 2005;27:843–50. [124] Michal SA, Adelstein DJ, Rybicki LA, et al. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head Neck 2012;34:1147–52. [125] Nguyen NP, Vock J, Chi A, et al. Impact of intensity-modulated and imageguided radiotherapy on elderly patients undergoing chemoradiation for locally advanced head and neck cancer. Strahlenther Onkol 2012;188:677–83. [126] Colevas AD. Chemotherapy options for patients with metastatic or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 2006;24:2644–52. [127] Cohen EE, Lingen MW, Vokes EE. The expanding role of systemic therapy in head and neck cancer. J Clin Oncol 2004;22:1743–52. [128] Gillison TL, Chatta GS. Cancer chemotherapy in the elderly patient. Oncology (Williston Park) 2010;24:76–85. [129] Schneider M, Thyss A, Ayela P, Gaspard MH, Otto J, Creisson A. Chemotherapy for patients aged over 80. In: Fentiman IS, Monfardini S, editors. Cancer in the elderly. New York: Oxford University Press; 1994. p. 53–60. [130] Repetto L. Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol 2003;1:18–24. [131] Karamouzis MV, Grandis JR, Argiris A. Therapies directed against epidermal growth factor receptor in aerodigestive carcinomas. JAMA 2007;298:70–82. [132] Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567–78. [133] Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21–8. [134] Jensen AD, Bergmann ZP, Garcia-Huttenlocher H, Freier K, Debus J, Munter MW. Cetuximab and radiation for primary and recurrent squamous cell carcinoma of the head and neck (SCCHN) in the elderly and multi-morbid patient: a single-centre experience. Head Neck Oncol 2010;2:34. [135] Alongi F, Bignardi M, Garassino I, et al. Prospective phase II trial of cetuximab plus VMAT-SIB in locally advanced head and neck squamous cell carcinoma. Feasibility and tolerability in elderly and chemotherapy-ineligible patients. Strahlenther Onkol 2012;188:49–55. [136] Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 2005;23:8646–54. [137] Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359(11):1116–27. [138] Stewart S, Cohen E, Licitra L, et al. A phase III randomized parallel-group study of gefi tinib (Iressa) versus methotrexate (IMEX) in patients with recurrent squamous cell carcinoma of the head and neck. Annual Meeting of the American Association of Cancer Research (AACR). Los Angeles, USA. April 14–18; 2007 [Abstr 3522]. [139] Argiris A, Ghebremichael M, Gilbert J, et al. Phase III randomized, placebocontrolled trial of docetaxel with or without gefitinib in recurrent or metastatic head and neck cancer: an eastern cooperative oncology group trial. J Clin Oncol 2013;31(11):1405–14. [140] Vermorken JB, Stohlmacher J, Davidenko I, et al. Primary efficacy and safety results of spectrum, a phase 3 trial in patients (pts) with recurrent and/or metastatic (r/m) squamous cell carcinoma of the head and neck (SCCHN) receiving chemotherapy with or without panitumumab (PMAB). Ann Oncol 2010;21:12. [141] Vermorken J, Stöhlmacher J, Oliner K, et al. Safety and efficacy of panitumumab (pmab) in HPV positive (+) and HPV negative ( ) recurrent/ metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): Analysis of the phase 3 spectrum trial, The 2011 European Multidisciplinary Cancer Congress; 2011.
© Copyright 2025