Homework Assignment for CHEM 4511 Professor J. M. Weber
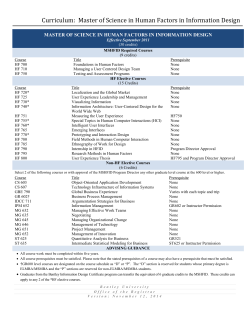
Homework Assignment for CHEM 4511 Professor J. M. Weber Office: JILA A709; Phone 303-492-7841; email: weberjm@jila.colorado.edu ________________________________________________________________________ Problem Set 11 To be returned before class on Wednesday, April 22, 2015. Please slide it under my office door or bring it to class. 1. Rate Laws a.) Calculate the integrated rate law for the product concentration in the unimolecular reaction A P. Start from the rate law to do so, and assume that there is no product present at time zero. (4 credits) b.) N2O decomposes according to 2 N2O(g) 2 N2(g) + O2(g). Under certain conditions, the rate of the reaction is 6.1610-6 molL-1s-1. Calculate the values of d[N2O]/dt, d[N2]/dt and d[O2]/dt. c.) The reaction (3 credits) SO2Cl2(g) SO2(g) + Cl2(g) is first order and has a rate constant of kr = 2.2410-5 s-1 at 320C. Calculate the halflife of the reaction. What fraction of the reactant remains after being heated for 5.00 hours at 320C? How long will a sample of SO2Cl2 need to be maintained at 320C for decomposition of 92% of the initial amount present? (3 credits)` d.) In biochemistry, radioactive labeling is often used in kinetics experiments and as markers in gel electrophoresis. You order a sample of Na3PO4 containing the radioisotope 32P (t1/2 = 14.3 days). If the shipment in transit is delayed for two weeks, how much of the initial activity will remain when you finally receive the sample? (3 credits) e.) In geology and archaeology, potassium-argon dating is used to date sedimentary rocks. 40K decays by two pathways: 40 K 40Ca + electron 40 K 40Ar + positron The overall half-life of 40K is 1.3109 years. Estimate the age of sedimentary rocks with a 40Ar:40K ratio of 0.0102. (4 credits) 1 Homework Assignment for CHEM 4511 Professor J. M. Weber Office: JILA A709; Phone 303-492-7841; email: weberjm@jila.colorado.edu ________________________________________________________________________ A products f.) Consider a reaction d [ A] kr [ A]n , dt that obeys the rate law where n is the reaction order and can have any value except n = 1. Show that for such a reaction the half-life is given by t1 / 2 1 1 2 n1 1 · · . kr n 1 [ A]0n 1 (4 credits) 2. Simulating Reaction Kinetics Getting integrated rate laws can be mathematically challenging even for relatively simple reactions, particularly if there are consecutive reactions, or if reactions are close to equilibrium. In this problem, you will learn how to set up simulations of reaction kinetics without resorting to integrated rate laws. This is easy to set up in Microsoft Excel or any similar program. The setup instructions here are for Excel. Start by populating the first few cells of an Excel sheet as shown below: 1 A Reaction B C AB 2 3 Rate Law vr = k[A] t [A] D E F G t / s 1 [A]0 / mol 1 k / s‐1 0.1 [B]0 / mol 0 k‐1 / s‐1 0.1 4 5 H [B] You will now set up a simulation for a very simple unimolecular reaction of the type AB which is first order in both directions. The rate constants are called k for the forward reaction and k-1 for the back reaction. 2 Homework Assignment for CHEM 4511 Professor J. M. Weber Office: JILA A709; Phone 303-492-7841; email: weberjm@jila.colorado.edu ________________________________________________________________________ Populate the first three cells in row 6 as follows: 0 =$H$1 =$H$2 The first entry gives the time at the start of the reaction (here defined as zero), and the other two entries define the concentrations at time zero. Now enter in the first three cells in row 7 the following: Into cell A7: =A6+$E$1 increases time by t as defined in cell E1 Into cell B7: =B6-$E$2*B6*$E$1+$E$3*$E$1*C6 according to [A]previous + [A] = [A]previous – k·[A]previous·t + k-1·[B]previous·t Into cell C7: =C6-$E$3*$E$1*C6+$E$2*$E$1*B6 according to calculates [A] at this time calculates [B] at this time [B]previous + [B] = [B]previous + k·[A]previous·t – k-1·[B]previous·t Now copy and paste the contents of cells A7, B7 and C7 into the cells below, so you get to 50 time steps total (populate all up to row 56). Now you have calculated the concentrations of A and B for 50 time steps. You can now play with the initial conditions (cells H1 and H2), the rates (cells E2 and E3) and the time resolution of the simulation (cell E1). Plot the concentrations of A and B to get the plot shown below: OK, now you are (hopefully) ready to tackle the following problems: 3 Homework Assignment for CHEM 4511 Professor J. M. Weber Office: JILA A709; Phone 303-492-7841; email: weberjm@jila.colorado.edu ________________________________________________________________________ a.) Change the initial concentration of B to 1 mol. Describe in one sentence what happens. (1 credit) b.) Change the initial concentration of B to 0.5 mol. What are the concentrations of A and B in the limit of t? (2 credits) c.) Set the initial concentration of B to zero. Now increase both rates in steps of 0.1 s-1. At what value do you stop getting meaningful results? Why (one sentence)? What can you do to make the simulation meaningful again, keeping the same rates (one sentence)? (4 credits) d.) Now, you will take the next step: on a new sheet, implement a simulation for the 2A B. reaction This is 2nd order in the forward direction and 1st order in the backward direction. You can check your results on the way by doing two things: i. Make a new column that contains the sum of the concentrations of A and B. This column must always be the same as the sum of the concentrations at time zero. ii. For long times, the equilibrium constant K must be the ratio of the forward and backward rate constants. The concentrations must behave accordingly. Set the initial concentration of A to 1 mol, B to zero, t to 1 s, and use 0.1 s-1 for both rates. What are the final concentrations for A and B (3 significant figures)? Plot the graph of your simulation. (6 credits) e.) Use the same settings as in (e), except for the initial concentration of B, which you set to 0.2 mol. What are now the final concentrations of A and B (3 significant figures)? Plot the graph of your simulation. (2 credits) f.) Use the following settings: t = 2 s; k = 0.1 s-1; k-1 = 0.01 s-1; [A]0 = 1 mol; [B]0 = 0. What are the final concentrations of A and B (3 significant figures)? Plot the graph of your simulation. (2 credits) We will continue with more simulations in the last problem set of the semester (PS 12), so please make every effort to get this working. 4
© Copyright 2025