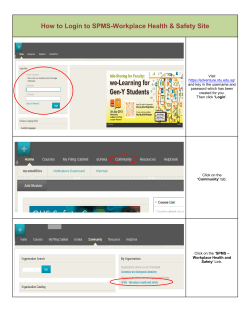
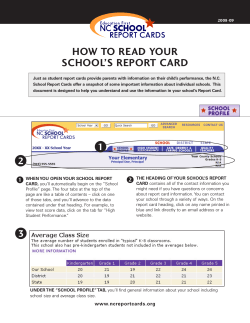
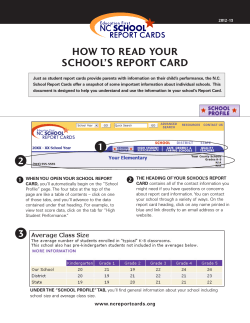
NIH S -2-S G
Office of Sponsored Projects NIH SYSTEM-2-SYSTEM GUIDE SAMPLE R01 PROPOSAL Coeus 4.5.1.X | Document Version 2│Updated January 2014 Table of Contents GUIDE OVERVIEW ................................................................................................................................................................... 2 o CONVENTIONS USED IN THIS GUIDE............................................................................................................................................. 2 NEW/ UPDATED GRANTS.GOV FORMS (FORMS – C) .................................................................................................... 3 o UPDATED PHS 398 APPLICATION FORMS, INSTRUCTIONS AND APPLICATION GUIDES o MODIFICATIONS TO NIH’s PLANNED AND CUMULATIVE INCLUSION ENROLLMENT FORMS ................................................... 4 ........................................... 4 SF424 (R&R) COVER V2-0 ........................................................................................................................................................ 5 PROJECT/PERFORMANCE SITE LOCATION(S) V2-0................................................................................................................ 11 RESEARCH & RELATED OTHER PROJECT INFORMATION V1-3 .............................................................................................. 14 RESEARCH & RELATED SENIOR/KEY PERSON PROFILE (EXPANDED) V2-0 ............................................................................ 18 PHS 398 COVER PAGE SUPPLEMENT V2-0 ............................................................................................................................ 21 PHS 398 RESEARCH PLAN V2-0 ............................................................................................................................................. 25 RESEARCH & RELATED BUDGET V1-3 .................................................................................................................................... 28 PHS 398 MODULAR BUDGET V1-2 ........................................................................................................................................ 38 R & R SUBAWARD BUDGET ATTACHMENT(S) FORM V1-3 ................................................................................................... 41 PLANNED ENROLLMENT REPORT FORM V1-0 ............................................................................................................ 43 PHS 398 CUMMULATIVE INCLUSION ENROLLMENT REPORT FORM V1-0 4.5.1.X – NIH S2S Guide – Sample R01 Proposal .................................................................. 45 1 GUIDE OVERVIEW The NIH S2S Guide provides detailed information on forms required for NIH proposal submissions. The guide describes each form and fields within those forms as well as where each field is located within Coeus. For detailed instructions on how to complete each field in Coeus, see the Coeus User Guides found at: http://www.brown.edu/research/coeusguides o CONVENTIONS USED IN THIS GUIDE Item Convention Note of interest Note image Tip Light bulb image New Functionality/Enhancement New sign Items that are in YELLOW boxes with RED outline are required information for Grants.gov validations! Example *Please Note – There may be other fields required by the Sponsor; refer to the specific Notice of Opportunity for details. Items that are in GRAY boxes are NOT modifiable by the end-user and are centrally maintained by the Office of Sponsored Projects! Information that is ITALICIZED IN RED in the R&R Forms indicates NIH Specific Instructions. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 2 NEW/ UPDATED GRANTS.GOV FORMS (FORMS – C) NIH has transitioned to updated grants.gov form packages (FORMS - C) starting with applications due on or after September 25, 2013. Exceptions: • Small Business Programs (SBIR/STTR) applicants will transition to Forms-C packages later in 2014. List of Updated Grants.gov Forms: SF424 (R&R) Cover PHS 398 Cover Page Supplement R&R Other Project Information PHS 398 Career Development Award Supplemental Form R&R Senior/Key Person (Expanded) PHS Fellowship Supplemental Form Project/Performance Site Location(s) PHS 398 Research Training Program Plan R&R Budget (5 Year & 10 Year) and Associated Subaward Budget PHS 398 Training Subaward Budget Attachment(s) PHS 398 Research Plan List of New Grants.gov Forms: Planned Enrollment Report PHS 398 Cumulative Inclusion Enrollment Report SF424 (R&R) Multi-Project Cover List of Retired Grants.gov Forms: PHS Cover Letter PHS 398 Checklist Changes in Coeus to Support FORMS-C Packages: o o o o o New Fields New Narrative Types New Window to upload Planned Enrollment Report and PHS 398 Cumulative Inclusion Enrollment Report Forms Updated Grants.gov Questions Questionnaire Modified Coeus to Grants.gov Forms Mapping 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 3 o UPDATED PHS 398 APPLICATION FORMS, INSTRUCTIONS AND APPLICATION GUIDES New application instructions and forms for paper-based PHS 398 applications and new application guide instructions for electronic SF424 (R&R) applications are now available: • • Paper-based PHS 398 Applications: http://grants.nih.gov/grants/funding/phs398/phs398.html Electronic SF424 (R&R) Application Guides: http://grants.nih.gov/grants/funding/424/index.htm Some of the changes include: • Supplemental Grant Application Instructions – is now a separate guide. (Parts II and III have therefore been removed from the PHS398 Application Instructions and the general Application Guides for Forms-B and Forms-C application packages.) • Notice of Proprietary Information – Clarifies the policy regarding the final determination of whether an application contains proprietary information and that a positive finding of proprietary information does not automatically shield the information from release in response to a Freedom of Information Act (FOIA) request should the application result in an award. • Biographical Sketch – “Patent Citations” have been added as part of section C, Selected Peer Reviewed Publications. • Letters of Support – Clarification on the required content. • Appendix – Photographs or color images of gels, micrographs, etc. are no longer accepted as Appendix material. Such images must now be included in the Research Strategy. • PHS 398 Applicants – All PHS 398 paper application submissions intended for due dates on or after September 25, 2013, must use the 8/2012 version of the PHS 398 instructions and forms. In addition, in the Research Plan, Inclusion Enrollment Reports have been renamed and modified and are now “Planned Enrollment Report” and “Cumulative Inclusion Enrollment Report”. • SF424 (R&R) Applicants – Applications using electronic forms will transition to new forms identified with a Competition ID of FORMS-C. In addition, The SF424 (R&R) guide contains a new Chapter - Supplemental Instructions to the SF424 (R&R) for Preparing a Multi-Project Application. The new application instructions and forms must be used for applications intended for due dates on or after September 25, 2013. Any application submitted using incorrect application forms (including applications that have an incorrect mix of old and new forms) may be delayed and may not be reviewed. o MODIFICATIONS TO NIH’s PLANNED AND CUMULATIVE INCLUSION ENROLLMENT FORMS NIH has modified forms used to provide information regarding planned enrollment and actual cumulative enrollment of individuals involved in clinical research studies on the basis of sex/gender, rate, and ethnicity. Key changes include: • The Planned Enrollment Report Form will now include the “More than one race” category. • The layout of both the Planned Enrollment Report and the Cumulative Inclusion Enrollment Report Forms has been modified to reduce confusion about racial and ethnic information being distinct concepts. • The Planned Enrollment Report and Cumulative Inclusion Enrollment Report are now structured data forms. • The modified reporting forms will be phased in starting with competing applications submitted electronically following the timeline of NIH’s use of the updated electronic applications (FORMS-C). Non-competing continuation applications will continue to use the current forms for inclusion data provided in the PHS 2590 or RPPR until further notice. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 4 SF424 (R&R) COVER V2-0 Box # 1 Adobe Forms Field Location COEUS Section Type of Submission Grants.gov Submission Details (Action→Grants.gov ) Dated Submitted 2 Applicant Identifier Date Received by State State Application Identifier 3 Grants.gov Submission Details (Action→Grants.gov) Proposal Details Screen N/A N/A COEUS Tab COEUS Field Name Instructions/Notes Opportunity Tab Submission Type Select from the drop-down menu. List options are: Pre-application, Application, and Change/Corrected Application. (System will default to “Application”) Submission Status Tab Received Date Date is inserted by COEUS upon OSP submission to Grants.gov Proposal Tab Proposal No. N/A N/A N/A N/A The unique identifying number of the Proposal Development record; assigned by COEUS. This field is not required This field is not required (Will be populated with the State of Rhode Island) New applications leave it blank unless submitting a NEW application following a Pre-Application or Instructed by Sponsor to complete. *Please read your specific opportunity instructions to understand what data and format should be provided in the Federal Identifier field. Further details about Sponsor specific guidelines for this field are located at the end of this section. Enter the agency-assigned routing identifier per agency-specific instructions. (Unless specifically Agency Routing Agency Routing 4b Proposal Details Proposal Tab noted in a program announcement, the Agency Identifier Identifier Routing Identifier is not used by NIH and other PHS agencies but is used for DOD submissions.) Previous Change/Corrected applications require an entry Previous Grants.gov 4c Proposal Details Proposal Tab Grants.gov in the Previous Grants.gov Tracking ID field. (See Tracking ID Tracking ID Change/Corrected Application Instructions) 4a 5 Proposal Tab Sponsor Proposal No. Organizational DUNS Organization Tab (Double click on the Proposal Organization) ↓ Organization-2 Tab Duns Legal Name Organization Tab (Double click on the Proposal Organization) ↓ Maintain Organization Window ↓ Name & Address Tab Organization Name Department/Davison Proposal Tab Lead Unit Address (Street, City, State, Zip Code) Organization Tab Proposal Organization Address/Contact Federal Identifier Proposal Details Proposal Details 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Users do not need to enter. The University Organization Data is stored in COEUS and maintained centrally by OSP. Lead Unit (Department) of the proposal is inserted into both, the Department & Division field (Not Modifiable). Users do not need to enter. The University Organization Data is stored in COEUS and maintained centrally by OSP. 5 Box # Adobe Forms Field Location 5 cont. Person to be contacted on matters involving this application 6 Employer Identification (EIN) or (TIN): 7 Type of Applicant Type of Application 8 If Revision, mark appropriate box(es): Is the application being submitted to other agencies? What other Agencies? 9 10 COEUS Section Proposal Details Proposal Details Proposal Details Screen Grants.gov Submission Details Investigator Tab (Double click on the Unit Name for the PI) ↓ Administrators Tab Organization Tab (Double click on the Proposal Organization) ↓ Organization-2 Tab 7 Type Tab in Maintain Organization Window Proposal Tab Opportunity Tab COEUS Field Name Electronic Submission Contact Federal Employer Questionnaire (Edit → Questionnaire) Proposal Details Screen Catalog of Federal Domestic Assistance Number Proposal Details Screen / Grants.gov Submission Details (Action→Grants.g ov) 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Instructions/Notes Users do not need to enter. Electronic Submission Contact is assigned to Proposal Lead Unit; this information is maintained by OSP. *If the contact is wrong, please contact OSP to correct. Section 5. Applicant Information now includes the additional contact information that was included on the PHS 398 Cover Page Supplement Form Users do not need to enter. The University Organization Data is stored in COEUS and maintained centrally for the University. Type Description Proposal Type Revision Type (Action→Grants.g ov) Name of Federal Agency Title COEUS Tab Select from the list: New, Resubmission, Renewal, Continuation, or Revision. Select from: Increase Award, Decrease Award, Increase Duration, Decrease Duration, or Other. (if “Other” need to specify in the box that populates) Questions for Grants.gov S2S Forms Is this application being submitted to other agencies? (If yes, need to provide explanation) If yes, search for and select the Acronyms of the other agency the application is being submitted to in the follow up question. Proposal Tab Sponsor This field will populate based on selected Sponsor. Proposal Tab CFDA Number Proposal Tab Opportunity Title The CFDA Number will populate base on the Grants.gov selected opportunity. (Field may be blank if applying for an opportunity that references multiple CFDA numbers) The Title will populate base on the Grants.gov selected opportunity. 6 Box # 11 12 Adobe Forms Field Location COEUS Section COEUS Tab Descriptive Title of Applicant’s Project Proposal Details Screen Proposal Tab Proposed Project: Start Date Proposed Project: End Date Proposal Details Screen Proposal Tab 13 Congressional District of Applicant Proposal Details Screen Organization Tab Double click on the Proposal Organization ↓ Name & Address Tab in Maintain Organization window Box # Adobe Forms Field Location COEUS Section COEUS Tab 14 Project Director/Principal Investigator Contact Information Proposal Details Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Investigator Tab COEUS Field Name Title Instructions/Notes Enter brief descriptive title of the project. 200 character limit (including spaces). (A “New” application must have a different title from any other PHS project with the same PD/PI. A “resubmission” or “renewal” application should normally have the same title as the previous grant or application. If the specific aims of the project have significantly changed, choose a new title. A “revision” must have the same title as the currently funded grant.) Start Date Enter the project Start Date. End Date Enter the project End Date. Congressional District Users do not need to enter. The University Organization Data is stored in COEUS and maintained centrally by OSP. COEUS Field Name Instructions/Notes Principal Investigator (indicated by the PI checkbox) Contact data and address details for the PI come from the Person Details of the PI. To edit the information go to: Edit → Proposal Personnel and then double click on the PI. You can modify the Department by changing the Unit Number. Directory Department information cannot be modified. 7 Box # 15a Adobe Forms Field Location Total Non-Federal Funds Requested 15c Total Federal & NonFederal Funds Requested 15d Estimated Program Income 17 COEUS Tab Total Federal Funds Requested 15b 16 COEUS Section Is this application subject to review by state under Executive order 12372 Process? By signing this application, I certify (1) to the statements contained in the list of certifications* and (2) that the statements herein are true, complete and accurate to the best of my knowledge. I also provide the required assurances* and agree to comply with any resulting terms if I accept an award. I am aware that any false, fictitious, or fraudulent statements or claims may subject me to criminal, civil, or administrative penalties. COEUS Field Name Instructions/Notes Total Costs Detailed Budget – Total Costs of all budget periods will be inserted. If there is no budget, the field will be set to zero. Modular budget – Total Direct Costs and Indirect Costs for the Entire Project Period in Cumulative tab of Modular Budget will be inserted. Cost Sharing The sum of total Cost Share committed will be inserted. If there is no cost share, this field will be set to zero. (The figure includes Cost Shared Overhead) Total Costs + Cost Sharing The Sum of Total Costs and Cost Share of all budget periods will be inserted. If there is no budget, this field will be set to zero. Total Program Income The Program Income will be inserted. If there is no program income, this field will be set to zero. Is this proposal subject to review by state executive order 12372 process? (YES) – This pre-application/application was made available to the State under the Executive Order 12372 Process for review on ___________ (Insert the date in the follow up question) (NO) – Select either: o Program is not covered by E.O. 12372; or o Program has not been selected by state for review. Budget Summary Tab Budget Section Edit ↓ Project Income Questionnaire (Edit → Questionnaire) OSP/BMRA Approval 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Questions for Grants.gov S2S Forms Final OSP/BMRA Approver (submitter) This field will be checked upon final approval. 8 Box # Adobe Forms Field Location COEUS Section 18 SFLLL (Disclosure of Lobbying Activities) or Other Explanatory Documentation) Narrative for Proposal Section 19 20 21 Authorized Representative OSP/BMRA Approval Map Pre-application Narrative for Proposal Section Cover Letter Attachment Narrative for Proposal Section 4.5.1.X – NIH S2S Guide – Sample R01 Proposal COEUS Tab COEUS Field Name Instructions/Notes Narrative Type: RRSF424_SFLLL_OtherE xplanatory If applicable, attach SFLLL (Standard Form LLL, Disclosure of Lobbying Activities) or other explanatory documentation per agency instruction. Final OSP/BMRA Approver (submitter) Narrative Type: Pre-application Narrative Type: RRSF424_Cover_Letter Users do not need to enter. The fields will be populated with the information specific to the OSP/BMRA approver and the time stamp of the approval. While routing for approval, this field displays the SO for the OSP/BMRA. If applicable, upload a pdf file. Unless specifically noted in a program announcement, NIH and other PHS agencies do not use Pre-applications. Users must prepare a document containing the required information and upload it as a pdf file. Applicants are encouraged to include a cover letter with the application. The cover letter is only for internal use and will not be shared with peer reviewers. A separate PHS Cover Letter form will no longer be used. However, NIH will continue to keep the Cover Letter separate from the assembled application image and available only to authorized staff. 9 FIELD 4A: Federal Identifier Field – NIH Grant Submissions: -For a “Continuation”, “Resubmission “, “Renewal”, or “Revision” Type of Application, enter the NIH IC and serial number of the previously assigned application/award number (e.g., CA987654) [Even if submitting a Change/Corrected Application] • Resubmissions: the IC/Serial number is in the prior Coeus Proposal record, in the Grants.gov panel, Submission Details tab, Agency Tracking ID field. • Renewals and Revisions: the IC/Serial number is part of the Sponsor Award Number and should be located in the Coeus Award. Otherwise, confirm this number at NIH eRA Commons. * Federal Identifier Field – DOE Grant Submissions: For a Renewals or Revisions to the Department of Energy, a DOE Project Identifier is often required. Follow the opportunity instructions for the data to insert in the Sponsor Proposal No. field in Coeus Proposals. Notes on Cover Letter: a. Check your announcement instructions for instances when the cover letter is a requirement. b. Required for any submission after the submission deadline, including submission to correct errors/ warnings within the “error correction window” that follows the submission deadline. If revising the cover letter for a changed/corrected application, include all previously submitted cover letter information. The system only retains the last cover letter submitted. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 10 PROJECT/PERFORMANCE SITE LOCATION(S) V2-0 Project/Performance Site Primary Location Box # Adobe Forms Field Location COEUS Section COEUS Field Name Instructions/Notes I am submitting an application as an individual, and not on behalf of a company, state, local or tribal government, academia, or other type of organization. Organization Question Question # 30 (currently not in COEUS) No user action required to complete this form field at this time. DO NOT check this box. NIH only accepts applications from organizations. Proposal Details Screen Organization Tab Performing Organization (Institutional COEUS Data) Organization Name No additional user action required to complete this form field. DUNS Number Proposal Details Screen Organization Tab Double click on the Performing Organization ↓ Organization-2 Tab in the Maintain Organization window Duns No additional user action required to complete this form field. Street, City, State, Zip Code, Congressional District Proposal Details Screen Organization Tab Organization Contact Person Information is stored in COEUS and maintained centrally for the University. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal COEUS Tab 11 Project/Performance Site Location # Box # Adobe Forms Field Location COEUS Section COEUS Tab Instructions/Notes I am submitting an application as an individual, and not on behalf of a company, state, local or tribal government, academia, or other type of organization. COEUS Field Name Not Required Not Required Not Required No user action required to complete this form field at this time. DO NOT check this box. Organization Name Proposal Details Screen Organization Tab Other Organization or Performance Site The user selected organization or Rolodex ID details populate the fields. NOTE: Rolodex must have complete address for this form to validate DUNS Number Proposal Details Screen Organization Tab Double click on the Other Organization ↓ Organization-2 Tab in the Maintain Organization window Duns The user selected organization or Rolodex ID details populate the fields. NOTE: Rolodex must have complete address for this form to validate DUNS number cannot be added for Performance SITE entries.* Street, City, State, Zip Code, Congressional District Proposal Details Screen Organization Tab Address/Contact The user selected organization or Rolodex ID details populate the fields. NOTE: Rolodex must have complete address for this form to validate Proposal Details Screen Organization Tab Additional Project/Performance Sites ≤ 300 Additional Project/Performance Sites > 300 Narrative for Proposal Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Up to 29 distinct locations can be maintained on this form Narrative Type: Performance_Sites Users must prepare a document containing the address details for the sites not able to be detailed on the form. 12 ADDITIONAL USEFUL INFORMATION: For Other Organizations, the COEUS Organization Table will be searched and the returned results will include: • Location • Address • DUNS Number • Congressional District. (Modify the district by deleting returned result and adding new congressional district field) For Performance Site: • Location field must be manually entered • Rolodex is searched to provide address • Congressional District field must be added and typed in. (Use Add Cong District to supply additional districts) Save any entries or updated to the Organization screen. DUNS NUMBER CANNOT BE ADDED FOR PERFORMANCE SITE ENTRIES (DUNS number entry is OPTIONAL for performance sites) NOTE: Maintain ZIP+4 in your Rolodex addresses. If the zip code submitted is only 5 digits, not zip+4, the application will fail validations at Grants.gov and eRA Commons. Other Organization vs. Performance Site: DUNS numbers are not populated for Performance Sites. Performance Sites are populated from Rolodex entries which do not contain DUNS numbers. Other Organizations are populated from the Organization table, which can maintain the DUNS number. You may notice a yellow highlight on the empty DUNS number filed when the application is processed at NIH eRA Commons for Performance Sites. This does not fail validation at either Grants.gov or eRA Commons at this time. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 13 RESEARCH & RELATED OTHER PROJECT INFORMATION V1-3 Box # Adobe Forms Field Location COEUS Section COEUS Tab COEUS Field Name Instructions/Notes The YES box must be checked if activities involving human subjects are planned at any time during the proposed project at any Are Human Subjects Proposal Details Review Type: performance site, even if the proposed project is Special Review Tab Involved? Screen Human Subjects exempt from Regulations for the Protection of Human Subjects. If no, skip the remaining questions about Human Subjects. To answer YES to Human Subjects Involvement: Proposal Details Screen → Special Review Tab. Follow the instructions below to enter your special review details. COEUS will check the appropriate boxes in items 1 and 1.a, and input the FWA code (The Human Subject Assurance Number is stored in the Organization Tab – under Proposal Organization). 1. Click the drop-down box in the field labeled Special Review 2. Select the Review Type “Human Subjects” 3. Click the drop-down box in the field labeled Approval and select a status appropriate to the review. IF: a. The status is Pending, then all required information has been entered. (No date required) b. The status us Submitted, enter the date of the Regulatory Review in the Application Date field. c. The status is Approved, then a protocol number must be entered in the Protocol No. field and a date entered into the Approval Date field. d. The status is Exempt, the exempt code must be entered in the Comments field. Valid exemption codes are: E1, E2, E3, E4, e5, E6. If multiple exempt codes are required entries s should be separated by a comma only, NOT spaces (i.e. E1,E2). 1 Exemptions: Unless otherwise required by department or agency heads, research activities in which the only involvement of human subjects will be in one or more of the following categories are exempt from this policy: Exemption (1) - Research conducted in established or commonly accepted educational settings, involving normal educational practices, such as (i) research on regular and special education instructional strategies, or (ii) research on the effectiveness of or the comparison among instructional techniques, curricula, or classroom management methods. Exemption (2) - Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures or observation of public behavior, unless: (i) information obtained is recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subjects; and (ii) any disclosure of the human subjects' responses outside the research could reasonably place the subjects at risk of criminal or civil liability or be damaging to the subjects' financial standing, employability, or reputation. Exemption (3) - Research involving the use of educational tests (cognitive, diagnostic, aptitude, achievement), survey procedures, interview procedures, or observation of public behavior that is not exempt under Exemption 2, if: (i) the human subjects are elected or appointed public officials or candidates for public office; or (ii) federal statute(s) require(s) without exception that the confidentiality of the personally identifiable information will be maintained throughout the research and thereafter. Exemption (4) - Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects. Exemption (5) - Research and demonstration projects which are conducted by or subject to the approval of department or agency heads, and which are designed to study, evaluate, or otherwise examine: (i) Public benefit or service programs; (ii) procedures for obtaining benefits or services under those programs; (iii) possible changes in or alternatives to those programs or procedures; or (iv) possible changes in methods or levels of payment for benefits or services under those programs. Exemption (6) - Taste and food quality evaluation and consumer acceptance studies, (i) if wholesome foods without additives are consumed or (ii) if a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or agricultural chemical or environmental contaminant at or below the level found to be safe, by the Food and Drug Administration or approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture. To answer NO to Human Subjects Involvement: If there are no Human Subjects Special Reviews entered, the question will be answered with a checkmark in the “NO” box. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 14 Box # Adobe Forms Field Location Are Vertebrate Animals Used 2 COEUS Screen Proposal Details Screen COEUS Field Name Special Review Type COEUS Field Name Instructions/Notes Review Type: Laboratory Animal Care The YES box must be checked if activities involving vertebrate animals are planned at any time during the proposed project at any performance site. If no, skip the remaining questions about Vertebrate Subject. To answer YES to Vertebrate Animals Use: Proposal Details Screen → Special Review Tab. Follow the instructions below to enter your special review details. COEUS will check the appropriate boxes in items 2 and 2.a, and input the Animal Welfare Assurance Number (The Animal Welfare Assurance Number is stored in the Organization Tab – under Proposal Organization). 4. Click the drop-down box in the field labeled Special Review 5. Select the Review Type “Animal Usage” 6. Click the drop-down box in the field labeled Approval and select a status appropriate to the review. IF: e. The status is Pending, then all required information has been entered. (No date required) f. The status us Submitted, enter the date of the Regulatory Review in the Application Date field. g. The status is Approved, then a protocol number must be entered in the Protocol No. field and a date entered into the Approval Date field. To answer NO to Vertebrate Animals Usage: If there are no Animal Use Special Review entered, the question will be answered with a checkmark in the “NO” box. Note: Consistent with the requirements of the Animal Welfare Act [7 U.S.C. 2131 et seq.] and the regulations promulgated by the Secretary of Agriculture [9 CFR, 1.1-4.11], NSF requires that proposed projects involving use of any vertebrate animal for research or education be approved by the submitting organization’s Institutional Animal Care and Use Committee (IACUC) before an award can be made. IACUC approval must be received prior to an award. Questions regarding this requirement should be directed to the cognizant NSF Program Officer. For applications involving the use of vertebrate animals, sufficient information must be provided within the 15-page project description to enable reviewers to evaluate the choice of species, number of animals to be used, and any necessary exposure of animals to discomfort, pain or injury. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 15 Box # 3 4a Adobe Forms Field Location COEUS Screen COEUS Tab Is proprietary/privileged information included in the application? Does this Project Have an Actual or Potential Impact – positive or negative – on the environment? COEUS Field Name Instructions/Notes Is proprietary/privileged information included in the application? If the application includes such information, check the “YES”, otherwise, check the “NO” box. Does this Project Have an Actual or Potential Impact on the environment? 4b If yes, please explain Please provide a brief explanation of the actual or potential impact on the environment. 4c If this project has an actual or potential impact on the environment, has an exemption been authorized or an Environmental Assessment (EA) or an Environmental Impact Statement (EIS) been performed? If this project has an actual or potential impact on the environment, has an exemption been authorized or an Environmental Assessment (EA) or an Environmental Impact Statement (EIS) been performed? 4d If yes, please explain 5a Is the research performance site designated, or eligible to be designated, as a historic place? 5b (Edit → Questionnaire) If yes, please explain 6a Does this project involve activities outside of the United States or partnerships with International Collaborators? If yes, identify countries 6b Optional Explanation 6 Questionnaire 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Questions for Grants.gov S2S Forms Please enter additional details about the EA or EIS. Is the research performance site designated, or eligible to be designated, as a historic place? Provide a brief explanation for the research performance site designated or eligible to be designated as a historic place. Does this project involve activities outside of the United States or partnerships with International Collaborators? Identify the countries. Provide a brief explanation for involvement with outside entities. To respond to the question, check YES, or NO. If you answered YES, enter an explanation (up to 55 characters) for the actual or potential impact on the environment in follow up question. To respond to the question, check YES, or NO. If you answered YES, enter a brief explanation (up to 55 characters) in the follow up question and/or upload the information in a separate file under the “Other Attachments” option. To upload: Edit ↓ Narrative Narrative Type: Other (please enter attachment title in the Module Title Field) To respond to the question, check YES, NO. If you answered YES, enter a brief explanation in the follow up question. To respond to the question, check YES, NO. If you answered YES, enter the names of the countries with which international collaborative activities are involved and enter brief explanation in the follow up question. *If you answered yes, applicants to the NIH and other PHS agencies MUST describe special resources of the research project, whether similar research is being done in the United States and whether there is a need for additional research in his area. Provide this information in a separate file; attaching is as Item 12, Other Attachments. To upload: Edit → Narrative; Narrative Type: Other Module Title Field: enter “Foreign_Justification” 16 Box # Adobe Forms Field Location COEUS Section COEUS Tab COEUS Field Name 7 Project Summary/Abstract Narrative for Proposal Section Narrative Type: Project_Summary 8 Project Narrative Narrative for Proposal Section Narrative Type: Narrative 9 Bibliography & References Cited Narrative for Proposal Section Narrative Type: Bibliography 10 Facilities & Other Recourses Narrative for Proposal Section Narrative Type: Facilities 11 Equipment Narrative for Proposal Section Narrative Type: Equipment 12 Other Attachments Narrative for Proposal Section Narrative Type: Other Instructions/Notes Users must prepare a document containing the required information and upload it as a pdf file. *This section must be no longer than 30 lines of text, and follow the required font and margin specifications. An abstract which exceeds this allowable length may be flagged as an error by the agency upon submission. System will give an error if over 1 page. Users must prepare a document containing the required information and upload it as a pdf file. *Using no more than two or three sentences, describe the relevance of this research to public health. In this section, be succinct and use plain language that can be understood by a general, lay audience. System will give an error if over 1 page. Users must prepare a document containing the required information and upload it as a pdf file. *Unless otherwise noted in an FOA, this section is required for submissions to NIH and other PHS agencies. Users must prepare a document containing the required information and upload it as a pdf file. * No special form is required but this section must be completed and attached for submissions to NIH and other PHS agencies unless otherwise noted in an FOA. Requires a description of how the scientific environment will contribute to the probability of success of the project, etc. Users must prepare a document containing the required information and upload it as a pdf file. For each upload, title/description is required. Users must prepare documents containing the required information and upload it as a pdf file. NOTE: COEUS will generate and display Field 12 only when the narrative type “Other” is uploaded in the proposal, otherwise this field description will not appear on the COEUS generated form. Multiple lines will be generated, as requires, for each upload. Questions in fields 4, 5, and 6 of this form allow for optional uploads to this location. *Please read your Sponsor Specific Opportunity instructions for the required content and page limits for all of the required attachments! 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 17 RESEARCH & RELATED SENIOR/KEY PERSON PROFILE (EXPANDED) V2-0 Box # Adobe Forms Field Location Profile - Project Director/Principal Investigator COEUS Section Proposal Details Screen COEUS Tab Investigator Tab COEUS Field Name Instructions/Notes PI Checkbox The individual that has the PI Checkbox checked off will be the one that will populate Project Director/Principal Investigator Section of this form. The PI Contact data stored in COEUS will populated the required information. Use the “Find Person” function to locate the Institute PI in the COEUS Person Data Table. Use the “Find Rolodex” function to locate non-employee individuals. In the Proposal Details Screen under the Investigator Tab/Key Person Tab, enter the estimated total project effort in the appropriate % effort field (% effort over the 12-month period) and check the Multi PI checkbox (if appropriate) prior to saving. Other field data returned from the search can be modified on the Details Screen (Edit → Proposal Personnel → Double Click on the PI or click on the PI and go to Edit → Person Details) Specific contact (phone, fax, commons user name, unit) and degree details may be edited for the submission in the above descried “Person Details” function for the person. Fields with white background are editable. Users can enter data in empty fields, or change existing data. Changes will only be made to this proposal and any copies of this proposal. To revert to the maintained COEUS-Institutional data, delete the investigator and then search and save the investigator again. COEUS DATA FOR THE PI WILL AUTOMATICALLY POPULATE FORMS UNLESS MODIFIED IN THE PERSON DETAILS SCREEN Prefix N/A N/A N/A This information does not populate the form. Name information of the individual responsible for the overall scientific and technical direction of the project. Same information as on the SF424 (R&R) form box 14. First Name Middle Name Last Name Proposal Details Screen Investigator Tab/Key Person Tab First Name Middle Name Last Name Suffix N/A N/A N/A This information does not populate the form. Proposal Details Screen Investigator Tab ↓ Edit → Proposal Personnel ↓ Double Click on the PI ↓ Primary Title Position/Title of the individual responsible for the overall scientific and technical direction of the project. Field editable in COEUS Premium only. Position/Title 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 18 Organization Tab in Person Details Window Organization Name Organization Tab Department/Divisi on Investigator Tab ↓ Edit → Proposal Personnel ↓ Double Click on the PI ↓ Organization Tab in Person Details Window Street1 Street 2 City County State Province Country Zip/Postal Code Phone Number E-Mail Proposal Details Screen Fax Number Credential, e.g., agency login Project Role/Other Project Role Category Investigator Tab ↓ Edit → Proposal Personnel ↓ Double Click on the PI ↓ Contact Info Tab in Person Details Window Investigator Tab ↓ Edit → Proposal Personnel ↓ Double Click on the PI ↓ Organization Tab in Person Details Window Investigator Tab ↓ Edit → Proposal Personnel ↓ Double Click on the PI ↓ Contact Info Tab in Person Details Window Investigator Tab/Key Person Tab Proposal Organization Organization name of the individual responsible for the overall scientific and technical direction of the project. Defaults to Brown University. Home Unit & Division Department of the individual responsible for the overall scientific and technical direction of the project. Can be edited by changing the Home Unit # on the “Person Details Screen” under “Organization Tab”. The Division information defaults to the Investigator’s Department and can be modified. Address 1 Address 2 City County State/Province Name Country Zip/Postal Code Address information for the PD/PI. These fields can be edited. Note: Make sure the Postal Code is NINE (9) digits. Office Phone Email Address Field can be edited. Fax Field can be edited. This field can be edited. Agency Credentials Check boxes Or “Role” Field (Key Person Tab) For NIH and other PHS agencies, registration in the eRA Commons for all PD/PIs is required. The Commons User Name must be entered here. This is a required field for applications submitted to NIH and other PHS agencies. Applications will not pass agency validation requirements without this field. For Principal Investigator – under the Investigator Tab the “PI Checkbox” must be checked. Other individuals under the investigator tab that do not have any boxes checked will be given Project Role of “Co-Investigator”. Under the Key Person Tab – the roles of the individual are typed in the “Role” Field. Highest academic or professional degree of credentials of the PD/PI. Field can be edited. Investigator Tab ↓ Degree Type Edit → Proposal Personnel ↓ Select the Individual Year the highest degree or other credential ↓ Degree Year Graduation Date was obtained. Edit → Degree Info Field can be edited. Degree Details Window Opens To Maintain Degree Details in COEUS Premium: Select Edit → Proposal Personnel; Select the Person requiring revisions or entries; Select Edit → Degree Info. Click Add to enter a new Degree; complete the entries and the click OK to save. Repeat if more than one degree is needed. Degree Type To Maintain Degree Details in COEUS Lite: On the Details Screen, select Add Degree; Select a Degree Type from the drop-down list; Enter the Degree, Graduation Year, and School in the fields provided. Repeat is more than one degree is needed. Save. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 19 Attach Biographical Sketch Attach Current & Pending Support Proposal Details Screen Investigator Tab ↓ Edit → Proposal Personnel; Proposal Personnel Screen ↓ Edit ↓ Add Module Module Document Type: Biosketch Module Document Type: Current and Pending To Upload the Biographical Sketch and Current and Pending Support: Select Edit → Proposal Personnel; Select the Person requiring the attachment; Select Edit → Add Module. Select Biosketch from the Document Type drop-down list. Click on the Description Field and enter a name for the attachment, then select Edit → Upload Attachment; select the appropriate document to upload. *Please read your Sponsor Specific Opportunity instructions for the required content and page limits for all the required attachments! ADDITIONAL SENIOR AND KEY PERSONS The Entries for all the Investigators and Key Persons are similar to the Principal Investigator requirements. Follow the instructions for maintaining the PI (above) and refer to the instructions in your selected funding opportunity and/or sponsor submission guidelines for specific requirements. IF YOU WOULD LIKE TO ARRANGE THE PERSONNEL IN A SPECIFIC ORDER PLEASE GO TO: PROPOSAL PERSONNEL WINDOW AND USE THE 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 20 PHS 398 COVER PAGE SUPPLEMENT V2-0 Box # 1 Adobe Forms Field Location COEUS Section COEUS Tab Prefix N/A N/A First Name Middle Name Last Name Proposal Details Screen Investigator Tab Suffix N/A N/A Questionnaire Agency-Defined Phase III Clinical Trial (Edit → Questionnaire) 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Questions for Grants.gov S2S Forms Instructions/Notes Does not populate the form. First Name Middle Name Last Name This form will be completed by COEUS for each individual listed in the Investigator Tab (For each co-PD/PI). Does not populate the form. Does this project include a Clinical Trial? Clinical Trial? 2 COEUS Field Name Does the project include a Phase III clinical trial? Check “YES” or “NO” to indicate whether the project is a clinical trial. Check “YES” or “NO” to indicate whether the project is an NIH-defined Phase III Clinical trial. (An NIH-defined Phase III clinical trial is a broadly based prospective Phase III clinical investigation, usually involving several hundred or more human subjects, for the purpose of either evaluating an experimental intervention in comparison with a standard or control intervention or of comparing two or more existing treatments. Often the aim of such investigation is to provide evidence leading to a scientific basis for consideration of a change in health policy or standard of care. The definition includes pharmacologic, non-pharmacologic, and behavioral interventions given for disease prevention, prophylaxis, diagnosis, or therapy. Community trials and other population-based intervention trials are also included. 21 Box # 3 4 Adobe Forms Field Location *Disclosure Permission Statement *Program Income COEUS Section Questionnaire (Edit → Questionnaire) Display Budget for Proposal Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal COEUS Tab Questions for Grants.gov S2S Forms Edit ↓ Project Income COEUS Field Name Instructions/Notes Disclosure Permission Statement If this application does not result in an awards, is the Government permitted to disclose the title of the proposed project, and the name, address, telephone number and e-mail of the official signing for the applicant organization, to organizations that may be interested in contacting you for further information? Check “YES” or “NO” to indicate whether disclosure permission is granted. Income Details Enter Income and Description for each project period with income. If application is funded, the Notice of Grant Award will provide specific instructions regarding the use of such income. The number of program income budget periods must be less than or equal to the number of periods included in the budget. 22 Box # Adobe Forms Field Location COEUS Section COEUS Tab COEUS Field Name Questions for Grants.gov S2S Forms Does the proposed project involve human embryonic stem cells? Can a specific stem cell line be referenced at this time? If stem cells will be used, but a specific line cannot be referenced at the time of application submission, include a statement that one from the registry will be used. List the registration number of the specific cell line(s) from the stem cell registry Human Embryonic Stem Cells 5 Specific stem cell line cannot be referenced at this time. One from the registry will be used. Questionnaire (Edit → Questionnaire) Cell Lines 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Instructions/Notes Check “YES” or “NO” to indicate whether Human Embryonic Stem Cells are used. Check “YES” or “NO”. If so, list the 4-digit registration numbers of the specific line(s) from the NIH Human Embryonic Stem Cell Registry in the follow up question. If a specific line cannot be referenced at the time of application submission, check the box that states “Specific stem cell line cannot be referenced at this time. One from the registry will be used.” Error if provided human embryonic stem cell lines are not listed at http://grants.nih.gov/stem_cells/registry/cu rrent/htm at time of submission. New form allows for collection of up to 200 stem cell lines (previous limit was 20) 23 Box # Adobe Forms Field Location COEUS Section *Inventions and Patents Questionnaire 6 (Edit → Questionnaire) COEUS Tab COEUS Field Name Instructions/Notes Questions for Grants.gov S2S Forms Is this a Renewal Application? Check “No” if no inventions were conceived or reduced to practice during the course of work under this project. Check “Yes’ if any inventions were conceived or reduced to practice during the previous period of support If “Yes” indicate Yes or No as to whether this information has been reported preciously to the PHS or to the applicant organization official responsible for patent matters. For Renewals ONLY: Check “YES” if there are inventions and they have been previously reported; Check “NO” if inventions have not been previously report; Check “N/A” is no inventions at all *Previously Reported Box # Adobe Forms Field Location 7 Change of principal investigator / program director Name of former principal investigator / program director COEUS Section Questionnaire (Edit → Questionnaire) Change of Grantee Institution Name of former institution 4.5.1.X – NIH S2S Guide – Sample R01 Proposal COEUS Tab Questions for Grants.gov S2S Forms COEUS Field Name Instructions/Notes Does this application reflect a change in principal investigator/program director from that indicated on a previous application? Check “YES” or “NO”. If ‘YES’ complete the follow up question. Search and select the former PD/PI Does this application reflect a change in grantee institution from that indicated on a precious application? Search and select the former Institution from the Organization records. Check “YES” or “NO”. If ‘YES’ complete the follow up question. 24 PHS 398 RESEARCH PLAN V2-0 Box # Adobe Forms Field Location 1. COEUS Section COEUS Tab COEUS Field Name Narrative Type: PHS_ResearchPlan_Intro ductionToApplication Introduction to Application Instructions/Notes For Resubmissions or Revisions only. Users must prepare a document containing the required information and upload it as a pdf file. Limited to ONE page unless specified in the FOA. NOTE: Introduction of Resubmission applications for R25 applications is limited to THREE pages. 2. Specific Aims Narrative for Proposal Section Narrative Type: PHS_ResearchPlan_Speci fic Aims (Edit → Narrative) 3. *Research Strategy Narrative Type: PHS_ResearchPlan_Rese arch Strategy 4. Progress Report Publication List Narrative Type:PHS_ResearchPlan_ ProgressReportPublicatio nList 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Users must prepare a document containing the required information and upload it as a pdf file. Limited to ONE page. Users must prepare a document containing the required information and upload it as a pdf file. Organize in the following order: Significance, Innovation, and Approach. Follow the page limits for the Research Strategy in the instructions or the specific FOA. For Renewal Applications only. Users must prepare a document containing the required information and upload it as a pdf file. 25 Human Subject Sections Box # Adobe Forms Field Location 5. Inclusion of Women and Minorities 7. Inclusion of Children Other research Plan Sections Vertebrate Animals COEUS Tab COEUS Field Name Narrative Type: PHS_ResearchPlan_ ProtectionOfHuman Subjects Narrative Type: PHS_ResearchPlan_I nclusionOfWomenA ndMinorities Narrative Type: PHS_ResearchPlan_I nclusionOfChildren Protection of Human Subjects 6. 8. COEUS Section Narrative for Proposal Section Narrative Type: PHS_ResearchPlan_ VertebrateAnimals (Edit → Narrative) Instructions/Notes Users must prepare a document containing the required information and upload it as a pdf file. This section is required for applicants answering “yes” to the question “Are human subjects involved?” on the R&R Other Project Information form and the research does not fall under Exemption 4. Users must prepare a document containing the required information and upload it as a pdf file. If Vertebrate Animals are involved in the project, address each of the five points listed in the PHS SF424 (R&R) Adobe Form Version C Application Guide Users must prepare a document containing the required information and upload it as a pdf file. 9. Selected Agent Research 10. Multiple PD/PI Leadership Plan 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Narrative Type: PHS_ResearchPlan_ SelectedAgentResea rch Select Agents are hazardous biological agents and toxins that have been identified by DHHS or USDA as having the potential to pose a severe threat to public health and safety, to animal and plant health, or to animal and plant products. CDC maintains a list of these agents. See http://www.cdc.gov/od/sap/docs/salist.pdf. Narrative Type: PHS_ResearchPlan_ MultiplePILeadershi pPlan Users must prepare a document containing the required information and upload it as a pdf file. For applications designating multiple PD/PIs, a leadership plan must be included. 26 Narrative Type: PHS_ResearchPlan_ ConsortiumContract ualArrangements 11. Consortium/Contrac tual Arrangements Users must prepare a document containing the required information and upload it as a pdf file. Explain the programmatic, fiscal, and administrative arrangements to be made between the applicant organization and the consortium organization(s). Users must prepare a document containing the required information and upload it as a pdf file. Narrative Type: PHS_ResearchPlan_ LettersOfSupport 12. Letters of Support Attach all appropriate letters of support, including any letters necessary to demonstrate the support of consortium participants and collaborators such as Senior/Key Personnel and Other Significant Contributors included in the grant application. Users must prepare a document containing the required information and upload it as a pdf file. 13. Resource Sharing Plan(s) Narrative for Proposal Section Appenidx (Edit → Narrative) 14. Appendix (if applicable) Narrative Type: PHS_ResearchPlan_ ResouceSharingPlan s Narrative Type: PHS_ResearchPlan_ Appendix NIH considers the sharing of unique research resources developed through NIH-sponsored research an important means to enhance the value and further the advancement of the research. When resources have been developed with NIH funds and the associated research findings published or provided to NIH, it is important that they be made readily available for research purposes to qualified individuals within the scientific community.(Data Sharing Plan, Sharing Model Organisms, Genome Wide Association Studies) Users must prepare a document containing the required information and upload it as a pdf file. The Narrative Type requires Description Title in the “Module Title” Field. (Do NOT use any special characters or spaces in make you type in COEUS) A maximum of 10 PDF attachments is allowed in the Appendix. If more than 10 appendix attachments are needed, combine the remaining information into attachment #10. Note that this is the total number of appendix items, not the total number of publications. A summary sheet listing all of the items included in the appendix is encouraged but not required. When including a summary sheet, it should be included in the first appendix attachment. *Please consult the sponsor instructions for your specific opportunity regarding the content requirements and the page limits for all the uploaded attachments. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 27 RESEARCH & RELATED BUDGET V1-3 TIPS: To preview what budget categories you cost elements are mapped under use the following feature located under menu item: View ↓ Customize Click the “Grouped By Category” option and then click on the “Apply” button. You will be able to view the budget period in the Category View: OR Check the “Category” box in Show Columns, and then click on the “Apply” button. (You will most likely need to resize the columns displayed in your window to see the Category Column) 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 28 Box # Adobe Forms Field Location Organizational DUNS Name of Organization Budget Type Start Date End Date A. COEUS Section COEUS Tab COEUS Field Name Proposal Details Screen Organization Tab Proposal Organization N/A N/A N/A Budget for Proposal Screen Period 1 Tab Start Date End Date Instructions/Notes Users do not need to enter. The University Organization Data is stored in COEUS and maintained centrally for the University. COEUS defaults to “Project” Requested/Proposed start date for budget period. Senior/Key Person First Name Middle Name Last Name Budget for Proposal Screen Project Role Proposal Details Screen Investigator Tab/Key Person Tab Budget for Proposal Screen Base Salary Cal. Months Acad. Months Sum. Months Budget for Proposal Screen Budget for Proposal Screen Period 1 Tab Edit → Persons “Budget Persons” opens Edit → Persons “Budget Persons” opens Period 1 Tab Click on the line item category the Senior/Key Person falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up (Senior/Key Person should be listed there) Budget for Proposal Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Period 1 Tab • • Select the line item category and go to “Items” on the menu bar and select “Personnel Budget” Click on the “Add” button and select the individual they want to add and click “OK”. The person will be added to the “Personnel Budget Details” window for that specific line item. Investigator Tab: PI Co-PI or Co-Investigator Key/Person Tab: “Role” field Under the Key/Person Tab, the assigned role in the “Role” Field will populate this field. Individuals who are under the Investigator Tab and who are not assigned a PI or Co-PI role will default to a Co-Investigator Role. Base_salay_px There is a Base_salary_p field for every year of the project for every person listed in the Budget Persons Window. The base salary specified in these fields will populate the corresponding year the budget is requested for. Click on the line item category the Senior/Key Person falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up The information is computed from the following fields: Start Date, End Date, Period, %Charged, %Effort Requested Salary To add individual to a line category users need to: Click on the line item category the Senior/Key Person falls under COEUS automatically computes the Cal. Months, Acad. Months, and Sum. Months based on the information provided in the fields (Start Date, End Date, Period, %Charged, %Effort) in the “Personnel Budget Details” window. COEUS automatically calculates this number based on the information provided by the user [Base Salary (Budget 29 Fringe Benefits Budget for Proposal Screen Funds Requested Budget for Proposal Screen Period 1 Tab Period 1 Tab ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ “Salary” Field Click on the line item category the Senior/Key Person falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ “Cost” Field under the Employee Benefits line (bottom of the screen) Click on the line item category the Senior/Key Person falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ “Salary” field + “Cost” field [under the Employee Benefits line (bottom of the screen)] Persons Window), Start Date, End Date, Period, %Charged, %Effort (Personnel Budget Details Window)]. COEUS automatically calculates this number based on the rates stored in COEUS and the information provided by the user [Base Salary (Budget Persons Window), Start Date, End Date, Period, %Charged, %Effort (Personnel Budget Details Window)] This number is computed by COEUS by adding the “Salary” field and the “Cost” field [under the Employee Benefits line (bottom of the screen)] for the individual. Follow the Instructions above for all other Senior/Key Personnel Total Funds requested for all Senior/Key Persons in the attached file Narrative for Proposal Screen Total Senior/Key Persons Budget for Proposal Screen Additional Senior/Key Persons (Attachment) Narrative for Proposal Screen Period 1 Tab Narrative Type: Additional_Key_Persons Total Salary and Fringe Requested for Individuals included in that attachment Click on the lines item categories the Senior/Key Persons fall under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ The total of “Salary” field + “Cost” field for all Senior/Key Person included in the budget Narrative Type: Additional_Key_Persons If your COEUS budget includes/lists more than 8 Senior/Key Person, COEUS will automatically calculate their TOTAL salary and fringe and populate that number in this field on the Grants.gov form. COEUS automatically calculates this number by totaling the funds requested for all Senior/Key Person. If there are more than 8 Senior/Key Person included, COEUS will AUTOMATICALLY generate this attachment and include in the budget. *If you have more than 8 Senior/Key Person, EVRY TIME you preview the Grants.gov budget Forms, COEUS will generated a new attachment for additional senior/key person; however it will not delete the previously generated Additional Senior/Key person attachment. You have to go to the Narrative section and delete the modules that you do not need so that the correct attachment is included with the budget. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 30 Box # B. Adobe Forms Field Location COEUS Section COEUS Tab COEUS Field Name Instructions/Notes Quantity Field Number of personnel in that category. COEUS automatically totals the number of individuals that fall under that category and populates that number in this field. Other Personnel Post-Doctoral Associates Number of Personnel Cal. Months Acad. Months Sum. Months Requested Salary Budget for Proposal Screen Fringe Benefits Funds Requested Period 1 Tab Click on the line item category the PostDoctoral Associate falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up The information is computed from the following fields: Start Date, End Date, Period, %Charged Click on the line item category the PostDoctoral Associate falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ “Salary” Field Click on the line item category the PostDoctoral Associate falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ “Cost” Field under the Employee Benefits line (bottom of the screen) Click on the line item category the PostDoctoral Associate falls under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ “Salary” field + “Cost” field (Employee Benefits line) COEUS automatically computes the Cal. Months, Acad. Months, and Sum. Months based on the information provided in the fields (Start Date, End Date, Period, %Charged, %Effort) in the “Personnel Budget Details” window. The numbers in the fields represent the total for all the individuals in that line category. COEUS automatically calculates this number based on the information provided by the user [Base Salary (Budget Persons Window), Start Date, End Date, Period, %Charged, %Effort (Personnel Budget Details Window)]. The number is the field represents the total Requested Salary for all the individuals who are in that line category. COEUS automatically calculates this number based on the rates stored in COEUS and the information provided by the user [Base Salary (Budget Persons Window), Start Date, End Date, Period, %Charged, %Effort (Personnel Budget Details Window)]. The number is the field represents the total Fringe Benefits for all the individuals who are in that line category. This number is computed by COEUS by adding the “Salary” field and the “Cost” field [under the Employee Benefits line (bottom of the screen)] for the individual. The number is the field represents the total Funds Requested for all the individuals who are in that line category. *Follow the Instructions above for the rest of the “Other Personnel” (Graduate Students, Undergraduate Students, Secretarial/Clerical, other) 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 31 Total Number Other Personnel Total of Quantity Field COEUS automatically totals the number of individuals in the budget that fall under the “Other Personnel” category and populates that number in this field. Total Other Personnel Click on the lines item categories the Other Personnel fall under ↓ Items → Personnel Budget ↓ “Personnel Budget Details” window opens up ↓ The total of “Salary” field + “Cost” field for all Other Personnel included in the budget COEUS automatically calculates this number by totaling the funds requested for all “Other Personnel” Total of all line items that fall under the salary category COEUS automatically calculates this number by totaling the funds requested for ALL personnel in the budget. Budget for Proposal Screen Total Salary, Wages and Fringe Benefits (A+B) Box # C. Adobe Forms Field Location Equipment Description COEUS Section Period 1 Tab COEUS Tab COEUS Field Name Instructions/Notes The description for equipment line item MUST be entered in COEUS. This description will populate on the Grants.gov detail budget form. Budget for Per NIH: Equipment is defined as an item of Period 1 Tab Proposal property that has an acquisition cost of $5,000 or Equipment Line Item – Screen Funds Requested more (unless the organization has established Cost Field lower levels) and an expected service life of more than one year. Follow the above instructions for all other equipment as all Equipment must be budgeted using individual line items for EACH piece of equipment with a description entered in the description field. Equipment Line Item – Description Field Equipment item If you plan on purchasing more than 10 pieces of equipment and list all of them individually, COEUS will list 10 pieces of equipment on the Grants.gov form only. Anything else will be included in an attachment generated by COEUS. Equipment listed in the attachment is selected randomly by COEUS and not in the order that it was entered in the system. Total funds requested for all equipment listed in the attached file Narrative for Proposal Screen Total Equipment Budget for Proposal Screen Period 1 Tab Narrative Type: Additional_Equipment Total funds requested for the additional equipment listed in the attachment Total funds requested for all equipment listed in the attached file. Dollar amount for each item should exceed $5000. In Brown’s case the amount for each item should exceed $3000. Total of all Cost fields under every Equipment Line Item This number is computed by COEUS by adding all the costs in every equipment line category. If the space provided cannot accommodate all the equipment proposed, attach a file listing each additional Additional Equipment Narrative Type: item and the funds requested. Attachment Additional_Equipment COEUS will automatically generate this attachment if more than 10 pieces of equipment are listed in the budget. *If you have more than 10 Equipment items, EVRY TIME you saved the budget, COEUS will generated a new attachment for additional Equipment; however it will not delete the previously generated Additional Equipment attachment. You have to go to the Narrative section and delete the modules that you do not need so that the correct attachment is included with the budget. Narrative for Proposal Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 32 Box # D. 1 Adobe Forms Field Location Travel COEUS Section COEUS Tab Travel Domestic Line Item – Cost Field Domestic Travel Costs 2 Foreign Travel Costs Budget for Proposal Screen Period 1 Tab Travel Foreign Line Item – Cost Field Travel Domestic + Travel Foreign Line Items – Costs Fields Total Travel Cost E. COEUS Field Name Instructions/Notes Identify the total funds requested for domestic travel. Domestic travel includes Canada, Mexico, and US possessions. Enter the total funds requested for foreign travel. Foreign travel includes any travel outside of North America and/or US possessions. This number is computed by COEUS by adding all the costs for all travel. Participant/ Trainee Support Costs 1 Tuition/Fees/health Insurance 2 Stipends 3 Travel 4 Subsistence 5 Other NSF Participant Costs – Tuition Line Item Budget for Proposal Screen Number of Participants/Trainees Total participants/Trainee Support Costs 4.5.1.X – NIH S2S Guide – Sample R01 Proposal NSF Participant Costs – Stipend Line Item NSF Participant Costs – Travel Line Item Period 1 Tab NSF Participant Costs – Other Line Item Total of all the NSF Participant Cost Line Items List total funds requested for Participant/Trainee Tuition/Fees/Health Insurance. List total funds requested for Participant/Trainee Stipends. List total funds requested for Participant/Trainee Travel. List total funds requested for Participant/Trainee Subsistence. List and describe any other participant trainee funds requested. List the total number of participants/trainees. Total Funds requested for all trainee costs. 33 Box # F. 1 2 3 4 5 Adobe Forms Field Location Other Direct Costs COEUS Section COEUS Tab COEUS Field Name Materials and Supplies Total Costs of all Line Items that fall under the Materials and Supplies Category Publication Costs Total Costs of all Line Items that fall under the Publication Costs Category Consultants Services Total Costs of all Line Items that fall under the Consultants Services Category ADP/Computer Services Total Costs of all Line Items that fall under the ADP/Computer Services Category Subawards/Consortium /Contractual Costs Budget for Proposal Screen Period 1 Tab Total Costs of all Line Items that fall under the Subawards/Consortium /Contractual Costs Category Equipment or Facility Rentals/User Fees Total Costs of all Line Items that fall under the Equipment or Facility Rentals/User Fees Category 7 Alterations and Renovations Total Costs of all Line Items that fall under the Alterations and Renovations Category 8 -10 Other Total Costs of all Line Items that fall under the Other Costs Category 6 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Instructions/Notes In the budget justification, indicate general categories such as glassware, chemicals, animal costs, including an amount for each category. Categories less than $1,000 are not required to be itemized. The proposal budget may request funds for the costs of documenting, preparing, publishing, or otherwise making available to others the findings and products of the work conducted under the award. In the budget justification include supporting information. In the budget justification, identify each consultant, the services he/she will perform, total number of days, travel costs, and the total estimated costs. The cost of computer services, including computer-based retrieval of scientific, technical and education information may be requested. In the budget justification, include the established computer service rates at the proposing organization if applicable. List total funds requested for 1) all subaward/consortium organization(s) proposed for the project and 2) any other contractual costs proposed for the project. This line item should include both direct and indirect costs for all subaward/consortium organizations. List total funds requested for equipment or facility rental/user fees. In the budget justification, identify each rental user fee and justify. In the budget justification, itemize by category and justify the costs of alterations and renovations including repairs, painting, removal or installation of partitions, shielding, or air conditioning. Where applicable, provide the square footage and costs. Add text to describe any “other” direct costs not requested above. Use the budget justification to further itemize and justify. Use lines 8-10 for such costs as patient care and tuition remission. If requesting patient care costs, request inpatient and outpatient costs separately 34 Box # G. Adobe Forms Field Location Direct Costs Total Direct Costs (A thru F) H. Indirect Costs COEUS Section COEUS Tab COEUS Field Name Instructions/Notes Budget for Proposal Screen Period 1 Tab Direct Cost Field COEUS automatically calculates this number based on the information provided by the user and the information stored in COEUS. Indirect Cost Type Indirect Cost Rate (%) Budget for Proposal Screen Summary Tab OH Rate Type Rates ↓ Rates for Proposal Applicable Rate Field This number is the total of all items that are NOT classified as excluded from the F&A costs calculations. COEUS computes this number by summing all the items (salary, fringe, supplies, etc.) that are not classified as excluded from the F&A costs calculation. Indirect Cost Field COEUS automatically calculates this number based on the information and rates stored in COEUS and the information provided by the user. Organization Tab (Double click on the Proposal Organization) ↓ Organization 3 Tab Cognizant Auditor Field (double click on the name to get the contact information) Users do not need to enter. The Data is stored in COEUS and maintained centrally for the University. Period 1 Tab Total Cost Field COEUS automatically calculates this number based on the information stored in COEUS and the information provided by the user. N/A N/A Currently not mapped in COEUS Indirect Cost Base ($) Period 1 Tab Funds Requested ($) Total Indirect Costs Cognizant Federal Agency (Agency Name, POC name, and POC Phone Number) I. Proposal Details Screen J. Total Direct and Indirect Costs Total Direct and Budget for Indirect Institutional Proposal Costs (G+H) Screen Fee K. Budget Justification Fee Funds Requested N/A Indicate the type of cost (e.g., Salary & Wages, Modified Total Direct Costs, or Other [explain]). Also indicate if Off-site. If more than one rate/base is involved, use separate lines for each. The Rates are stored in COEUS and maintained centrally for the University. User must select the appropriate rates to be applied from the “Rates for Proposal” Screen. The rates can be modified by going into the “Rates for Proposal” Screen and typing over the rates that are already in there. Use the budget justification to provide the additional information requested in each Attach Budget budget category identified above and any Justification other information the applicant wishes to submit to support the budget request. *Follow the instructions described above for the rest of the Periods that you have requested funding for! Narrative for Proposal Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Narrative Type: S2S_Budget_Justificati on 35 Box # A B Adobe Forms Field Location Senior/Key Person Other Personnel COEUS Section COEUS Tab COEUS Field Name Budget for Proposal Screen Total Tab Total of all the “Total” fields of all the line items that fall under this category Total Tab Total of all the “Total” fields of all the line items that fall under the Senior/Key Person and Other Personnel categories plus the Employee Benefits Lines Total Number Other Personnel Total Salary, Wages and Fringe Benefits (A+B) Budget for Proposal Screen 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Instructions/Notes All values on this form are autocalculated. They present the summations of the amounts that you have entered previously for each of the individual budget periods. Therefore, no data entry is allowed or required, in order to complete this “Cumulative Budget” section. If any of the amounts displayed on this form appears to be incorrect, you may correct it by adjusting one or 36 C Equipment Travel D Domestic Travel Budget for Proposal Screen Total Tab Budget for Proposal Screen Total Tab Foreign Travel Tuition/Fees/health Insurance Stipends E Travel Subsistence Other Total of all the “Total” fields of all the Equipment line items Total of all the “Total” fields of all the Travel line items “Total” field of the Travel Domestic line item “Total” field of the Travel Foreign line item “Total” field of the Tuition/Fees (Fellow/Trainee) line item “Total” field of the Stipends (Fellow/Trainee) line item “Total” field of the Travel (Fellow/Trainee) line item “Total” field of the Subsistance (Fellow/Trainee) line item “Total” field of the Other (Fellow/Trainee) line item The number of Participants/Trainees that can be entered has been increased from 999 to 9999. Number of participants/Trainees F Materials and Supplies Total of all the “Total” fields of all the line items that fall under this category Publication Costs Total of all the “Total” fields of all the line items that fall under this category Consultant Services ADP/Computer Services Budget for Proposal Screen Subawards/Consortium /Contractual Costs Equipment or Facility Rental/User fees Alteration and Renovations G Other 1 Other 2 Other 3 Direct Costs (A thru F) H Indirect Costs I Total Direct and Indirect Costs (G+H) J more of the values that contribute to that total. To make any such adjustments, you will need to revisit the appropriate budget period, to enter corrected values. Fee 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Total Tab All values on this form are autocalculated. They present the summations of the amounts that you have entered previously for each of the individual budget periods. Therefore, no data entry is allowed or required, in order to complete this “Cumulative Budget” section. If any of the amounts displayed on this form appears to be incorrect, you may correct it by adjusting one or more of the values that contribute to that total. To make any such adjustments, you will need to revisit the appropriate budget period, to enter corrected values. Total of all the “Total” fields of all the line items that fall under this category Total of all the “Total” fields of all the line items that fall under this category Total of all the “Total” fields of all the line items that fall under this category Total of all the “Total” fields of all the line items that fall under this category Total of all the “Total” fields of all the line items that fall under this category Total of all the “Total” fields of all the line items that fall under this category “Total” field of the OH - MTDC line item “Total” field of the Total line item 37 PHS 398 MODULAR BUDGET V1-2 Modular budgets are applicable to certain research grant applications from domestic organizations requesting $250,000 or less per year for direct costs. International organizations and others that do not fall under this definition should use the detailed budget forms. Consortium/contractual F&A costs are not factored into the direct cost limit. They may be requested in addition to the $250,000 limit. Modular budgets are simplified; therefore, detailed categorical information is not to be submitted with the application. The modular budget is applicable only to R01, R03, R15, R21, and R34 applications. Note AHRQ does not accept modular budgets. For all modular budgets, request total direct costs (in modules of $25,000) reflecting appropriate support for the project. There will be no future year escalations. A typical modular grant application will request the same number of modules in each year. Provide an additional narrative budget justification for any variation in the number of modules requested. COEUS BUILDS THE MODULAR BUDGET FROM THE DETAILED BUDGET CREATED; THEREFORE THE DETAILED BUDGET IS ENTERED FIRST. Modular Budget Expenses are mapped to the Coeus Modular Budget screen from the Detailed Budget Version marked as Final. Modular expenses can be manually entered, or synchronized from the detailed budget. Adobe Forms Field Location Budget Period 1 Box # Start Date End Date A. COEUS Section Budget for Proposal Screen COEUS Tab COEUS Field Name Instructions/Notes Period 1 Tab Start Date End Date Requested/Proposed start date for budget period. This information is coming from the detailed budget that was entered. Direct Costs 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 38 Direct Cost less Consortium F&A Direct Cost less Consortium F&A Budget for Proposal Screen Consortium F&A Edit ↓ Modular Budget (Period 1 Tab) Total Direct Costs B. Consortium F&A Total Direct Costs Summary of the direct cost line items excluding subcontracts indirect (with Brown F&A and without Brown F&A) for that period. Total is rounded up to the nearest $25,000. This field can be edited. Total F&A costs included in the subcontract budgets for that period. (Not rounded) This field can be edited. COEUS automatically calculates this by adding the Direct Cost less Consortium F&A field + Consortium F&A field. Indirect Costs Indirect Cost Type Indirect Cost Type Indirect Cost Rate (%) IDC Rate (%) Indirect Cost Base ($) Budget for Proposal Screen Edit ↓ Modular Budget (Period 1 Tab) Funds Requested ($) Funds Requested Cognizant agency (Agency Name, POC Name and Phone Number) Proposal Details Screen Indirect Cost Rate Agreement Date Proposal Details Screen Total Indirect Costs Total Direct and Indirect Costs (A+B) Funds Requested ($) IDC Base Budget for Proposal Screen Organization Tab (Double click on the Proposal Organization and Maintain Organization Window opens) ↓ Organization 3 Tab Cognizant Auditor Field (double click on the name to get the contact information) Organization Tab (Double click on the Proposal Organization and Maintain Organization Window opens) ↓ Organization 3 Tab Edit ↓ Modular Budget (Period 1 Tab) Populated based on the detailed budget. Indicates the type of IDC base. This field can be edited. Populated based on the detailed budget. Indicates the rate of indirect cost applied This field can be edited. Total of the direct costs line items that accrue IDC. COEUS populates this field with the IDC Base from the Detailed budget. This field should be modified to be the IDC Base calculated from the Modular Budget. This is the Indirect Costs requested. COEUS automatically calculates this number based on the information provided. Cognizant Auditor Field Users do not need to enter. The Data is stored in COEUS and maintained centrally for the University. Indirect Cost Rate Agreement Field Users do not need to enter. The Data is stored in COEUS and maintained centrally for the University. Total Indirect Costs Total of all the Indirect Costs Funds Requested Auto calculated by COEUS. Funds Requested Total of the Total Direct Costs + Total of Indirect Costs. Auto-calculated by COUES. *Follow these instructions for all other Periods that the funding is requested for! 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 39 Box # 1. Adobe Forms Field Location COEUS Section COEUS Tab COEUS Field Name Edit ↓ Modular Budget (Cumulative Tab) Total Direct Cost less Consortium F&A for Entire Project Period Total Consortium F&A for Entire Project Period Total Direct Costs for Entire Project Period Total Indirect Costs for Entire Project Period Total Direct and Indirect Costs (A+B) for Entire Project Period Total Costs, Entire Project Period Total Direct Cost less Consortium F&A for Entire Project Period Total Consortium F&A for Entire Project Period Total Direct Costs for Entire Project Period Budget for Proposal Screen Total Indirect Costs for Entire Project Period Total Direct and Indirect Costs (A+B) for Entire Project Period 2. Instructions/Notes All values on this form are auto-calculated. They present the summations of the amounts that you have entered previously for each of the individual budget periods. Therefore, no data entry is allowed or required, in order to complete this “Cumulative Budget” section. If any of the amounts displayed on this form appears to be incorrect, you may correct it by adjusting one or more of the values that contribute to that total. To make any such adjustments, you will need to revisit the appropriate budget period, to enter corrected values. Budget Justification Narrative Type: PHS_ModBud_Per sonJustification Personnel Justification Consortium Justification Edit Additional Narrative Justification 4.5.1.X – NIH S2S Guide – Sample R01 Proposal Narrative Narrative Type: PHS_ModBud_Con sortiumJustificatio n Narrative Type: PHS_ModBud_Nar rativeJustification List ALL personnel, including names, number of person months devoted to the project (indicate academic, calendar, and/or summer) and roles on the project. Do not provide individual salary information. Provide an estimate of total costs (direct plus facilities and administrative) for each year, rounded to the nearest $1,000. List the individuals/organizations with whom consortium or contractual arrangements have been made, along with all personnel, including percent of effort (in person months) and roles on the project. Do not provide individual salary information. Indicate whether the collaborating institution is foreign or domestic. If the requested budget requires any additional justification, such as variations in the number of modules requested, include this information in this attachment. 40 R & R SUBAWARD BUDGET ATTACHMENT(S) FORM V1-3 Box # Adobe Forms Field Location Please attach Attachment 1 COEUS Screen Display Budget for Proposal Screen COEUS Tab Edit ↓ Sub Award COEUS Field Name Sub Award Budget Instructions/Notes A completed PDF R&R Subaward Budget Attachment form must be uploaded to the COEUS Premium Budget. NOTE: a complete Subaward budget is only required when the prime grantee is submitting a detailed budget using the R&R Budget Component. When completing the Project Role for the Investigator leading the portion of the project at the consortium site, the project role of “PD/PI” should only be used if the entire application is being submitted under the Multiple PI policy. Also, the role of Co-PD/PI is not currently used by NIH and other PHS agencies. Do not assign any individual this role. If applicants wish to use roles of “Co-Investigator” or “Consortium PI”, select “Other” for the Project Role field and then insert the appropriate role descriptor in the Other Project Role Category field. Using Correct R&R Subaward Budget Form in your Coeus S2S Proposal Given the various versions of R&R Subaward and R&R Budget forms available, we have developed the table below to help you determine on which R&R Budget Form to enter the Subaward Budget Details in order for Coeus to translate the Subaward Budget information correctly. Follow the usual steps to connect to Grants.gov in Coeus and in the Forms Tab of the Grants.gov Submission Details Window, include the checkbox for the RR Subaward Budget and make note of that specific attachment form name (e.g. RR Subaward Budget 30 V1.3). Then use the table below to determine which R&R Budget Form version the Subawardee (or you) must use to enter the Subaward Budget Details: If your S2S Application includes one to these Grants.gov Subaward Budget Attachment Forms… …use the following R&R Budget Form to enter the Subaward Budget information: RR SubAward Budget 30 V1.3 RR Budget V1-3 RR SubAward Budget V1.2 RR SubAward Budget 30 V1.2 RR Budget V1-1 RR Subaward Budget 10 10 V1.3 RR Subaward Budget 10 30 V1.3 RR Budget V1-3 RR Subaward Budget 10 10 V1.2 RR Budget10 V1-1 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 41 RR Subaward Budget 10 30 V1.2 RR FedNonFedSubawardBudget V1.2 RR FedNonFedSubawardBudget 30 V1.2 RR FedNonFedBudget V1.1 RR FedNonFedSubawardBudget 10 10 V1.2 RR FedNonFedSubawardBudget 10 30 V1.2 RR FedNonFedBudget10 V1.1 Note: It is always best practice to extract the required form directly from the Grants.gov Adobe Forms Package for the FOA that you are applying to! TO EXTRACT THE R&R SUBAWARD BUDGET ATTACHMENT: 1. Locate and download the appropriate opportunity from Gants.gov website 2. Open the Adobe opportunity form set 3. Select the subaward form and move it to the “FOR SUBMISSION” box to open the form 4. Click on the “Click here to extract the R&R Subaward Budget Attachment” button to extract the form 5. Save the file locally using the fist ten letters of the consortium oranization’s name and use “.pdf” as the file extension 6. Complete the required Budget entires (subwawrd budget requirements are identical to those of the proposal lead budget) → Save TO UPLOAD THE COMPLETED R&R SUBAWARD BUDGET ATTACHMENT TO THE PREMIUM PROPOSAL DEVELOPMENT BUDGET 1. Open the Proposal Details Screen 2. Edit 3. Select Budget → Click on the “Modify” button to bring you to the “Modify Budget for Proposal” Screen 4. Edit 5. Sub Award → “Sub Award Budget” window opens → Click on the “Add” button 6. Find the Subaward file, select it and click “Open” 7. Double click on the “Organization Name” field and enter the subcontractor’s name (no spaces or special characters) 8. Click on the “Translate” button (If the file has been generated successfully you should see the following: green checkmark in the “XML” field, a system generated name in the “Attachments” field and a message stating “XML Generated successfully” in the “Status” filed) 9. Click “OK” Button to close the window and return to the Budget 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 42 PLANNED ENROLLMENT REPORT FORM V1-0 Box # Adobe Form Name Planned Enrollment Report COEUS Screen User Attached S2S Forms (Edit → User Attached S2S Forms…) 4.5.1.X – NIH S2S Guide – Sample R01 Proposal COEUS Tab COEUS Field Name Instructions/Notes A completed PDF Planned Enrollment Report Form must be uploaded in this section when Proposal includes Human Subjects and is not E4 43 Where do I find the forms? Unstitched forms are provided by Grants.gov. They can be retrieved at the Grants.gov web page http://www.grants.gov/web/grants/forms/r-r-family.html#sortby=1 Forms with an asterisk (*) in the PDF column are unstitched and available for use with the upload tool. Note: the comment at the top of the table – that the forms are “not submittable” – means that they are not intended to be submitted individually to Grants.gov. These have been ‘unstitched’ to be included in our S2S submissions as part of a full submission. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 44 PHS 398 CUMMULATIVE INCLUSION ENROLLMENT REPORT FORM V1-0 Box # Adobe Form Name PHS 398 Cumulative Inclusion Report Form COEUS Screen User Attached S2S Forms (Edit → User Attached S2S Forms…) COEUS Tab COEUS Field Name Instructions/Notes A completed PDF PHS 398 Cumulative Inclusion Report Report Form must be uploaded in this section when Proposal includes Human Subjects and is not E4 Where do I find the forms? Unstitched forms are provided by Grants.gov. They can be retrieved at the Grants.gov web page http://www.grants.gov/web/grants/forms/r-r-family.html#sortby=1 Forms with an asterisk (*) in the PDF column are unstitched and available for use with the upload tool. Note: the comment at the top of the table – that the forms are “not submittable” – means that they are not intended to be submitted individually to Grants.gov. These have been ‘unstitched’ to be included in our S2S submissions as part of a full submission. 4.5.1.X – NIH S2S Guide – Sample R01 Proposal 45
© Copyright 2025