Peripheral IV and PICC Safety in Pediatric and Neonatal Patients
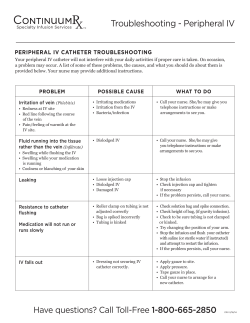
Peripheral IV and PICC Safety in Pediatric and Neonatal Patients Agenda Welcome and Overview Medical Device Adverse Event Reporting Through MedSun and KidNet The Influence of Human Factors Peripheral IV and PICC Catheter Safety Considerations in Pediatric and Neonatal Patients Case Studies The Importance of Reporting Medical Device Adverse Events MedSun 800-859-9821 medsun@s-3.com Nursing Continuing Education One contact hour of nursing continuing education credit is available Speakers Suzanne Rich, RN, MA, CT (moderator) Senior Project Manager, KidNet Office of Surveillance and Biometrics (OSB) Division of Patient Safety Partnerships (PSP) Center for Devices and Radiological Health (CDRH) Food and Drug Administration (FDA) Angela James, RN, RRT, BS Nurse Consultant OSB, PSP CDRH, FDA Speakers (continued) Dana Etzel-Hardman, RN, MSN, MBA, CPN Training and Education Specialist Children’s Hospital of Pittsburgh University of Pittsburgh Medical Center Jacqueline Francis, MD, MPH Medical Officer Plastic Surgery and Reconstruction Branch Office of Device Evaluation (ODE) CDRH, FDA MedSun 800-859-9821 medsun@s-3.com Speaker Angela James, RN, RRT, BS Nurse Consultant OSB, PSP CDRH, FDA Organization of FDA FDA is comprised of several Centers Three Centers deal with medical products: Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Center for Devices and Radiological Health (CDRH) MedSun KidNet Center for Devices and Radiological Health Center for Devices and Radiological Health (CDRH) CDRH promotes and protects the health of the public by ensuring the safety and effectiveness of medical devices and the safety of radiological products. MedSun An adverse event reporting program Over 350 health facilities participate The primary goal of MedSun is to work collaboratively with the clinical community to identify, understand, and solve problems with the use of medical devices. KidNet Focuses on identifying, understanding, and solving problems with medical devices used in neonatal and pediatric intensive care units (NICUs and PICUs) Launched in June 2007 40 participating MedSun hospitals “Medical errors most often result from a complex interplay of multiple factors. Only rarely are they due to the carelessness or misconduct of single individuals.” Lucian L. Leape, M.D. A leading patient safety expert from Harvard University Reported Adverse Events IV products (e.g. infusion pumps, pump tubing, PICCs and peripheral IV catheters) are the most frequently reported devices in MedSun reports involving pediatric/neonatal patients The top reported problems with both PICCs and peripheral IV catheters are associated with removal or replacement leaks breaks; cracks Examples of MedSun IV Device Adverse Events Packaging, product defects, product not performing as intended contaminants in the catheter packaging burr noted on needle prior to insertion guidewire became unraveled during insertion Problems that occurred during clinical use otoscope and transilluminator looked the same but had different light intensities; otoscope mistakenly used to illuminate vein for IV catheterization resulting in a patient burn inability to flush a PICC due to obstructed lumen resulted in embolization of a catheter fragment. PICC Luer hub cracked and leaked 15 “Swiss Cheese” Model of System Failure that Can Lead to Injury Device Maintenance Device Problem Reporting Culture of Low Expectations FAILURE 1 PICC Luer hubs cracking during clinical use. First leak occurs. Departmental Communication FAILURE 2 New PICC catheter inserted. Luer hub cracking problem not reported. FAILURE 3 Luer hub cracking problems continue to go unreported. FAILURE 4 Others not alerted to PICC Luer hub cracking problems Another PICC Luer hub crack occurs resulting in break and air embolism. 16 Case Study #1 Reported Luer Hub Cracks Several reports were received from a single MedSun KidNet site that described events of Luer hub cracking, leaking, and breaking. The problem was originally attributed to users over tightening the Luer hub connections. FDA follow up with both the KidNet reporting site and the manufacturer prompted the manufacturer to visit the site to better understand the reported events as they occurred during clinical use. The manufacturer subsequently made materials and design changes to address the reported events. 17 Human Factors Issues that Contribute to Medical Device Adverse Events Human Factors – the science of how humans interact with technology; focuses on the device-user interface, incorporates the following: Device Design Considerations Device-user interface, including labeling and instructions for use – auditory, visual, tactile Situations/Environment in which the Device is Used Light/noise intensity, time pressures, distractions, high stress User Characteristics Skills, training, expectations, familiarity with devices Policies and procedures for device use, cleaning, maintenance 18 Speaker Dana Etzel-Hardman, RN, MSN, MBA, CPN Training and Education Specialist Children’s Hospital of Pittsburgh University of Pittsburgh Medical Center 19 Major Complications with Peripheral Catheters Infiltration Extravasation Nerve injuries Hematomas Infection Phlebitis Cellulitis Occlusion Catheter Fracture Venous Spasm 20 IV Insertion Challenges IV insertion challenges with pediatric patients: Children’s anatomy – small, fragile, difficult to locate veins Patient pain, fear, anxiety Practitioner skill in IV insertion Some facilities have an IV team comprised of staff certified in IV insertion that assists bedside nurses (team members receive specialized training in accessing sites not used in adults, i.e., scalp or foot). 21 IV Insertion Tips Use age appropriate explanations Perform insertion outside patient’s room Allow child to keep comfort item (i.e. blanket) Encourage parent to be present Use age appropriate distraction techniques 22 Infant (birth-1 year) Educate the parent or caregiver on what to expect about reason for IV insertion and site appearance. Use comfort measures such as a pacifier if appropriate and try not to insert IV immediately after feeding to avoid aspiration. Utilize age appropriate distractions including games, songs, nursery rhymes. If a pedal IV site is chosen, get assistance to hold the infant’s foot in position. 23 Toddler (1-3 years) Provide simple, concrete explanations immediately before the procedure. Offer transitional objects such as stuffed toys for comfort. Distract with songs, games, or counting. Reward cooperative behavior with stickers or small toys. May require more than one person to help with positioning. 24 Preschool (3-4 years) Prepare child just before procedure. Encourage patient handling of, or medical play with some of the device equipment that may be used, e.g., placing an IV into a stuffed animal. Reward good behavior. Distract with concrete games, songs. 25 School Age (5-12 years) Explain procedures in simple terms. Encourage the child to help set up equipment or do small related tasks. Respect the child’s choice of whether or not to have parents present. Let the child know it’s o.k. if he or she is apprehensive or afraid. 26 Adolescents (13-20 years) Maintain privacy and assure confidentiality, recognizing adolescents may prefer that parents not be present. Explain procedure in adult language. Allow adolescents to participate in care decisions. Be aware of adolescents’ concern for body image in choice of vascular access device and care. 27 Parent Care Offer parents the option to attend the procedure Explain what to expect to parents Describe roles available for parents to play Reassure patient Observe Wait outside the room Assist with comfort measures 28 Peripheral Short-Term Catheter Selection From the Infusion Nurses Society (INS) 2006 Practice Guidelines: Choose the catheter size and type based on therapy A peripheral short-term catheter should be defined as one that is less than or equal to 3 inches (7.5 cm) in length Peripheral short-term catheters and steel-winged infusion sets should be equipped with a safety device with engineered sharps injury protection. 29 Catheter Placement Precautions Inspect the catheter for product condition prior to insertion. Care should be used when stylets, needles, and wires are used to facilitate catheter placement. Don’t reinsert stylets as there is a potential for severing or puncturing the catheter. A needle or guidewire should never be withdrawn through a catheter. 30 Case Study #2 Device Problems Noted Before Patient Use CDRH works with manufacturers to address reported problems and improve the product: When the package of an IV needle was opened, the plastic catheter fell off the needle hub. The needle was noted to be bent and retracted. Before use on patient, it was noticed that the catheter had been speared by the needle below the catheter tip. 31 IV Site Selection Site selection choice based on type and duration of IV therapy and patient development level INS guidelines (2006) for peripheral IV insertion indicates selecting veins in the dorsal and ventral surfaces of the upper extremities, including the metacarpal, cephalic, and basilic. Consider the patient's condition, age and diagnosis, vascular condition, history of previous access devices. For neonatal and pediatric patients, additional sites may include veins of the head and lower extremities. The vasculature should be able to accommodate the catheter size and length required for the prescribed therapy. 32 IV Site Preparation Clean site thoroughly prior to application of antiseptic solutions. Follow manufacturer indications and instructions for use when applying antiseptic agents Antiseptic solutions that should be used include: alcohol chlorhexidine gluconate (CHG) povidone-iodine ***Use as single agents or in combination, used individually, or in series. Formulations containing a combination of alcohol (ethyl or isopropyl) and either CHG or povidone-iodine are preferred. 33 IV Site Preparation CDC - category IA recommendation for 2% chlorhexidine-based preparation preferred over iodine or alcohol; FDA has not approved CHG for use in neonates younger than 2 months. 34 IV Site Access and Catheter Placement Warm the site Topical local anesthetic Tourniquet appropriate for child’s size Small gauge catheter INS recommends consideration of visualization technologies that aid in vein identification and selection. Vein viewer/transilluminator – a near infrared device to view veins Adverse events have been reported when using devices not approved for vein illumination 35 Case Study #3 Neonatal Transilluminator A two-month-old patient in the NICU received a second degree burn to the right forearm after a transilluminator was used to locate a vein for intravascular access. The reporting facility convened an interdisciplinary team with staff from risk management, engineering, and the NICU. Temperature testing where the light emitting diodes (LEDs) at the end of the transilluminator make contact with the patient’s skin, indicated that the device, over time, can become hot enough to cause thermal burns to fragile infant skin. MedSun/FDA follow up with the manufacturer resulted in a recall. The recall was for excessive heating due to incorrect wire assembly process. 36 Securing Catheters The pediatric patient population is likely to touch and manipulate catheters Secure the catheter to prevent occlusion, stress, kinking or migration. The highest rates of dislodgement were reported in patients 1 to 5 years old. Twiddler’s Syndrome – when kids play with the venous access device causing catheter to dislodge and/or infiltrate the site. Make sure that site is visible. Tubular elastic netting applied over a child’s arm may help prevent dislodgement. 37 Securing Catheters Although sutures, tape, and surgical strips are used to secure or stabilize catheters, the INS recommends use of a manufactured catheter-specific stabilization device when feasible. Follow the manufacturer's instructions for use. Remove the catheter stabilization device at established intervals to allow visual inspection of the access site and monitoring of skin integrity. Apply sterile tape or surgical strips only to the catheter adapter and don’t place directly on the catheter-skin junction site. 38 Case Study # 4 Securing Peripheral IVs A two-year-old was admitted to a local pediatric unit for dehydration secondary to diarrhea. An IV was started in her foot, to deliver fluid; potassium chloride was added to the IV solution once her urinary output was sufficient. Several hours after admission, a nurse noticed the IV site on the patient’s foot appeared to be discolored. When the large dressing was removed, a very large infiltration with marked circulatory compromise was seen. Emergency measures were implemented and the patient was transferred to a tertiary care center, where she underwent a fasciotomy in an attempt to restore her circulation. She had two additional surgical procedures, and two weeks later she was discharged home with extensive physical therapy and 39 plastic surgery follow up. IV Site Care and Assessment Assess IV site dressing and the entire IV administration set at the beginning of each shift and according to your facility’s policy/procedures. Assessment should include: visualizing and inspecting the site, checking the security and integrity of the dressing Follow your institution’s protocols for catheter site care. Use antiseptic solutions according to manufacturer directions. 40 Site Dressings The CDC recommends the use of either sterile gauze or a sterile transparent semi-permeable membrane (TSM) dressing to cover the catheter site. Advantages and Disadvantages of TSM dressings Enhances visualization for ongoing assessment and security of the catheter. Provides an artificial dermal layer, allows decreased moisture, and can be used for prolonged periods of time without damaging skin. However, there is a potential for damage to the stratum corneum and possible discomfort upon removal. Advantages and Disadvantages of Gauze Dressings Provides absorbency when there is moisture present (i.e., oozing at the insertion site) however they can be bulky and may require more 41 frequent dressing changes due to moisture retention. Peripheral IV and PICC Dressing Changes Pediatric and neonatal patients may experience some discomfort during removal of dressings. Minimize stimulation to the neonate using individualized approach according to gestational age and medical history Use age appropriate explanations for pediatric patients Containment strategy- positioning and/or restraints Oral sucrose for neonates, distraction for older patients Focused lighting on the procedure area – subtle lighting overhead Adhesive remover Dressing stretching techniques prior to removal 42 Speaker Jacqueline Francis, MD, MPH Medical Officer Plastic Surgery and Reconstruction Branch Office of Device Evaluation (ODE) CDRH, FDA 43 Common Complications with PICC Catheters Infection Infiltration/Extravasation Hematomas, Phlebitis, Venous Thrombosis Occlusion, Dislodgement, Air Embolism Catheter Fractures Potential complications include migration, perforation, arrhythmia, and embolization of catheter fragments. 44 PICC Catheter Selection Central vascular access devices have the distal tip dwelling in the lower one third of the superior vena cava to the junction of the superior vena cava and the right atrium. Make anatomical measurements to determine the length of the catheter needed and ensure appropriate catheter tip placement. If catheter is modified to a patient-specific length, document the inserted catheter length. 45 Preparation for PICC Insertion Maximal barrier precautions, including sterile gown, powder-free sterile gloves, cap, mask, protective eyewear, and large sterile drapes and towels should be used for midline and peripherally inserted central catheters, and all other central catheter insertions. 46 PICC Catheter Placement Check for adequate blood return prior to infusion of fluids. Radiographic confirmation of the central catheter's tip location needs to be obtained immediately after device insertion and in the following clinical situations: pain or discomfort after catheter placement; inability to obtain positive aspiration of blood; inability to flush the catheter easily; difficulty removing guidewire or it is bent upon removal; after catheter migration is noted. 47 Case Study #5 PICC Placement Problem A patient had a PICC line inserted at the bedside. There were no problems noted at the time of insertion. A post-procedure chest X-ray and follow up CT scan of the patient's chest showed a foreign body in the right subclavian vein. Later, it was determined to be a guidewire broken during placement and located in soft tissue. It could not be removed; thus it is considered a retained device fragment. 48 PICC Catheter Site Assessment and Care Use aseptic technique, including sterile gloves and a mask to clean the catheter-skin junction with an appropriate antiseptic solution, apply a stabilization device and apply a sterile dressing. Check insertion site and extremity for erythema or edema along the vein track. Document the length of externally lying catheter. A catheter that has migrated externally should not be readvanced prior to restabilization, regardless of stabilization or securement method. Check all tubing connections from the catheter to the infusion pump and verify correct pump alarm settings. 49 Case Study #6 PICC Catheter Assessment Reports of device related complications noted during PICC catheter assessments A PICC catheter became clotted and was unable to be used for fluid administration or to draw blood. The catheter was sluggish to flush and one port was totally occluded. The catheter was discontinued for use and a peripheral intravenous catheter was inserted. A PICC line catheter was leaking at the proximal end of the catheter. A split in the catheter was noticed at a seam point where the catheter leg connected to the proximal hub. The catheter was removed. 50 PICC Catheter Safety Tips Pounds per square inch (psi) is determined by syringe diameter and the amount of force applied to the plunger. Never use <10 cc syringes for flushes and medication administration Avoid twisting or kinking the catheter Do not flush against resistance or use excessive force Partial or complete catheter occlusions may be caused by thrombosis, drug precipitates or mechanical factors 51 Case Study #7 Catheter Storage A PICC was successfully placed in an infant through the femoral vein. Less than 24 hours later, the catheter broke, with a portion remaining inside the infant’s vascular system. After it was removed, a second PICC was inserted. Within minutes, it also broke for no apparent reason. The patient required an additional intervention to retrieve the second catheter segment. Both catheters were discovered to be hard and brittle. 52 Case Study #7 Catheter Storage What we learned: The facility stored the catheters, which come in transparent wrap, in a carousel inventory control system with a clear front panel that exposed them to ultraviolet (UV) light. The catheters were made of polyethylene, which is susceptible to degradation by UV light over time. Both catheters were close to their labels’ expiration dates. 53 Catheter Storage Safety Tips Follow manufacturer recommendations for storage Visually inspect catheter packaging and catheter/accessories for defects prior to placement Check expiration dates prior to catheter use Be familiar with manufacturer device-specific instructions for catheter maintenance Certain chemicals, solutions, or antibiotic ointments should not be used near catheters made of materials susceptible to damage 54 Your Role in Medical Device Patient Safety Recognize, report, and understand medical device problems Identify actual and potential problems, near misses, and potential for harm events with medical devices Report the problem or adverse event to your supervisor, according to policy and procedure Make sure your report includes details Remove the device and save the packaging 55 When Do I Report? When you think a device has, or may have, caused or contributed to any of the following outcomes for a patient, staff member, or visitor: Death Serious injury Minor injury Close-calls or other potential for harm 56 What Information Is Needed for a Report? If there was an injury, what happened to the person(s) affected? What were the problems with the device(s)? air embolism, respiratory arrest catheter leaking at hub What, if any, were the follow up medical procedures required because of the event? Surgery to remove retained catheter fragment Include the original procedure for which the device was used What is the name of the device manufacturer? What are the manufacturer device identification numbers? serial, model, lot, catalog, and any other specific information 57 Take Home Message Why Reporting Medical Device Problems Is A Model for Patient Safety In Your Hospital: Helps to prevent future medical device problems within your facility and protects patients, staff, families, and visitors by creating a climate of patient safety and also, Identifies actual or potential public health risks for the nation’s pediatric patients and their care providers because medical device adverse event information alerts FDA, manufacturers, and the clinical community to safety issues. 58 Questions and Answers What is the length of time a peripheral IV can be left in a pediatric patient? 59 Questions and Answers How do I know if a PICC can be used with a power injector? 60 Questions and Answers Where can I find information about FDA recalls relating to IVs and PICCs? New product approvals and clearances, recalls and safety alerts, and patient safety information http://www.fda.gov/MedicalDevices/default.htm MedSun monthly newsletter http://www.fda.gov/MedicalDevices/Safety/MedSun MedicalProductSafetyNetwork/default.htm 61 Question and Answers Clinical Practice Related Questions Infusion Nurses Society http://www.ins1.org/i4a/pages/index.cfm?pageid=1 Association for Vascular Access http://www.avainfo.org/website/article.asp?id=4 CDC http://www.cdc.gov/ The Joint Commission http://www.jointcommission.org/ 62 MedSun 800-859-9821 medsun@s-3.com 63 References Etzel-Hardman, Dana. (Nov. 2008) Teaching IV Therapy to Pediatric Nurses. RN. pp 24-28 INS Standards of Practice. (January/February 2006). Journal of Infusion Nursing. 29(1) Supplement, pp S1 – S62. MAUDE (Manufacturer and User Facility Device Experience database): http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/search.CFM Rich, Suzanne. (April 2009). KidNet: Get Set for Patient Safety. MedSun Newsletter # 35. Accessed 8-21-09. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/medsun/news/newsletter. cfm?news=35. Rosenthal, Kelli. (December 2005). Tips for Venipuncture in Children. Nursing. p 31. 64 References Sharpe, Elizabeth L. (June 2008). Tiny Patients, Tiny Dressings: A Guide to the Neonatal PICC Dressing Change. Advances in Neonatal Care. 8(3), pp. 150-162. Sullivan, Roberta. (Apr 2006). Light can Wreak Havoc on CVCs. MedSun Newsletter #3. Accessed 8-21-09. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/medsun/news/newslet ter.cfm?news=3#1 Sullivan, Roberta. (Feb 2006). Preventing PICC Fractures. MedSun Newsletter #1. Accessed 8-21-09. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/medsun/news/printer.c fm?id=644 65
© Copyright 2025