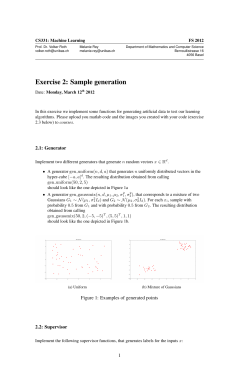
Available Projects - University of Birmingham
College of Medical and Dental Sciences Intercalated BMedSc Clinical Sciences Research projects 2015/16 Please state your preferences for up to six research projects from this booklet on your application form. You are expected to consult the supervisor(s) before submitting your application form. Application forms are available online: http://www.birmingham.ac.uk/students/courses/undergraduate/med/Cli nicalScienceBMedSc-IntercalatedDegree.aspx and should be returned to the course Administrator: Y.Palmer@bham.ac.uk or via post to: Miss Yvonne Palmer Room 220 Teaching Office School of Cancer Sciences University of Birmingham Vincent Drive Edgbaston Birmingham B15 2TT Ethical considerations We understand that students' ethical standards and convictions vary and may influence the type of work you feel you can conduct throughout the course. Students should be assured that all projects have ethics approval if required. However it is possible that personal convictions may be brought into conflict with the nature of the work you are conducting. For example some projects involve the use of cell lines that have been derived from human embryonic tissue. Other products involve use of animal matter and animal testing. Please talk directly to your supervisor from the outset about the exact nature of the materials, methods and procedures in the projects you are interested in. Home Office ‘Working with Animals’ Licence If your project requires you to attend this course, please ask your supervisor to book a place for you on the next course. Payment for a place on the course can be made from an existing account (your supervisor can confirm an account code) and reconciled once confirmation of the lab consumables account is received. Home Office Course Contact: J.e.penson@bham.ac.uk 2 College of Medical and Dental Sciences Intercalated BMedSc in Clinical Sciences Research projects for 2015/2016 Lead Supervisor: Dr Zubair Ahmed Contact Email: Telephone: z.ahmed.1@bham.ac.uk 0121 4148858 Co Supervisor: Prof Ann Logan Project Title: PI3K/Akt pathway-initiation of regeneration of the dorsal column projection of dorsal root ganglion neurons. Department: Neurobiology, CEM Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? No Project Outline Recent work has demonstrated a key role for mTOR signalling in axonal regeneration and the potential therapeutic utility in neurotrauma patients of next generation siRNA drugs that modulate the signalling pathway. Activation of PI3K/PDK/Akt pathway regulates axon growth cone dynamics through GSK3β, axogenic protein synthesis through mTORC1 and neuronal survival by activating anti-apoptotic pathways. Deletion of the phosphatase, PTEN, activates the PI3K pathway and leads to survival of axotomised neurons and axogenesis in the injured visual system1-3, corticospinal tracts (CST) in the cord4,5 and peripheral nerves5. This suggests that modulation of the mTOR pathway might be beneficial for promoting CNS axon regeneration after trauma. To date, there are no data on PTEN, Akt, mTOR and GSK3β expression in dorsal column (DC) axotomised DRGN, and the effects of siRNA knockdown of signalling molecules in the above pathways on DC axon regeneration have not been studied. In this project, we hypothesise that activation of the mTOR pathway is critical for DC axon regeneration. Therefore, we will modulate key components of the mTOR pathway both in vitro and in vivo using therapeutic siRNAs and monitor their effects on DC axon regeneration. Transganglionic tracers will detect regenerating axons in adult rats after DC injury as well as various markers to identify the injury response in longitudinal sections of the spinal cord. Confirmation of the pathways involved will be evaluated by microarray analysis of mRNA extracted from affected spinal ganglion neurons. These studies will yield important data on the use of therapeutic siRNAs to modulate the mTOR pathway and promote DC axon regeneration. References 1. Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. (2008) Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322:963-966. 2. Kurimoto T, Yin Y, Omura K, Gilbert H, Kim D, Cen L-P, Moko L, Kügler S, Benowitz LI. (2010) Long-distance axon regeneration in the mature optic nerve: Contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci 30:1565415663. 3. Leibinger M, Müller A, Gobrecht P, Diekmann H, Andreadaki A, Fischer D. (2013) 3 Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 4:e609;doi:1038/cddis.2013.126. 4. Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. (2010) PTEN deletion enhances the regeneration ability of adult corticospinal neurons. Nature Neurosci 13:1075-1081. 5. Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. (2013) Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord lesions. J. Neurosci. 33:15350-14361. How are you planning to ensure adequate supervision? Students will be supervised by Dr Ahmed on a day-to-day basis and will receive input from Prof Logan through weekly progress meetings. Students will also be paired up with other postdocs and senior PhD students who perform all of the routine techniques required for this project. The student role. The student will cut the sections of the spinal cord using a cryostat and then proceed to perform immunohistochemistry, microarray analysis and western blot. The student will also perform microscopy and analyse collected images using Image analysis software. Students will interact with other members of the laboratory who are working on related molecules involved in DC axon regeneration and partake in weekly lab meetings, where they will have a chance to present their work to colleagues from the Neurotrauma Research Group. 4 Lead Supervisor: Dr. Francesca Barone Contact Email: Telephone: f.barone@bham.ac.uk 07726899695/ 01213713248 Co Supervisor: Dr. Anne Fletcher and Dr. Saba Nayar Project Title: Lymphoid stromal cells: friends or foes? Department: Rheumatology Research Group Will the project require a Home Office working with animals licence? Yes Is the Project Cancer related? No Project Outline In secondary lymphoid organs (SLOs) such as lymph nodes and spleen, lymphoid stromal cells provide lymphocyte survival signals (such as IL-7, BAFF) and chemokine (CXCL13, CCL19, CCL21) cues. These allow migration of T and B cells and maintain SLO architecture and function(1, 2). Several distinct stromal cell populations have been identified in SLOs, including fibroblastic reticular cells (FRC) that sustain T cell survival and organization, follicular dendritic cells (FDC) that regulate antigen presentation and the germinal center (GC) reaction and marginal reticular cells (MRC) whose role remains unclear. These cell populations are characterized by different combinations of cell surface markers (gp38/Podoplanin, MAdCAM-1, VCAM-1, ICAM-1 and RANK-L) and differential expression of lymphoid chemokines and survival factors (3). Stromal cells can influence the size and shape of the immune repertoire, not only by modulating the availability of the survival factors, but also inducing deletion or expansion of specific cell clones. This process, ultimately aimed to induce peripheral tolerance, is achieved by stromal cells’ presentation of a range of peripheral tissue restricted antigens (4). Recent reports demonstrate that LN stromal cells also induce CD4+ T cell tolerance (5, 6). These data suggest that induction of tolerance is not achieved by a single cell type but by different stromal cells toward different peripheral tissue antigens. Indeed lymph node FRCs, upon inflammation, can produce nitric oxide (NO) a natural immunosuppressant, which influences T cell expansion and priming (7-9). 5 TLOs are accumulations of lymphoid cells that share similar cellular compartments, spatial organization, vasculature, chemokines and function to secondary lymphoid organs (10-14). Chronic antigenic stimulation is believed to be required for TLO maintenance, while tolerance seems not to occur. This suggests that locally activated TLO lymphoid like stromal cells might have lost the capacity to present antigens and express NO to induce tolerance and immunosuppression unlike their lymph node counterparts. Project objective: To explore immunosuppressive functions of lymphoid stromal cells in TLOs (tertiary lymphoid organs) compared to reactive lymph node stromal cells. Plan of Investigation: In order to compare the immunosuppressive potency of TLO-associated lymphoid stromal cells with lymphoid stromal cells from reactive secondary lymphoid organs particularly lymph nodes (LNs) we will isolate and study lymphoid like stromal cells from LN and TLO that form in the salivary glands of wild type mice infected with a replication deficient adenovirus. Adenovirus induced formation of TLOs in C57/BL6 SG (cannulation model). This model involves delivery of replication-defective adenovirus 5 (AdV5) via retrograde cannulation of submandibular gland excretory ducts in C57BL/6 mice (15). Within 15 days post cannulation TLO form that present T/B cell segregation and expression of lymphoid cytokines and chemokines. Mice will be immunized with NP-CGG Alum+Bordetella pertussis. This will involve injecting the paw-pad with a commonly used antigen [(4-hydroxy-3nitrophenyl)-acetyl conjugated to Chicken Gamma Globulin] NP-CGG Alum+Bordetella pertussis to activate local draining lymph nodes (LNs). TLO-associated lymphoid stromal cells will be isolated using a protocol developed in-house to isolate stromal cells from various organs such as salivary glands and lymph nodes. Phenotypical differences between TLO and SLO stroma will be studied by FACS analysis prior to sorting. On isolated, sorted stromal cells we will perform gene expression profile analysis for an array of immunosuppressive genes such as NOS2 (also known as iNOS i.e. inducible nitric oxide synthase), Arginase-1, IDO (indoleamine 2,3-dioxygenase), PD-L1, TGFβ, cyclooxygenases (COX-1 and COX2) and 6 lipooxygenases (LOX-5 and LOX-15). Once we have established the ex vivo immunosuppressive phenotype, stromal cells will be isolated from either (TLOs or LNs) and co-cultured with activated lymphocytes to assess the immunosuppressive function of stromal cells. As an indicator of immunosuppressive ability of stromal cells, T cell proliferation and the levels of inflammatory cytokine production by T cells will be determined. At the same time, culture supernatants will be used to evaluate the production of immunosuppressive molecules (such as Nitric oxide (NO), TGFβ, IL-10 and prostaglandin E2) from stromal cells. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. References Mueller SN, Germain RN. 2010. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Reviews Immunology Roozendaal R, Mebius RE. 2011. Stromal Cell–Immune Cell Interactions. Annual Review of Immunology 29: 23-43 Kain MJ, Owens BM. 2013. Stromal cell regulation of homeostatic and inflammatory lymphoid organogenesis. Immunology 140: 12-21 Fletcher AL, Malhotra D, Turley SJ. 2011. Lymph node stroma broaden the peripheral tolerance paradigm. Trends in Immunology 32: 12-8 Dubrot J, Duraes FV, Potin L, Capotosti F, Brighouse D, et al. 2014. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. J Exp Med 211: 1153-66 Abe J, Shichino S, Ueha S, Hashimoto S, Tomura M, et al. 2014. Lymph node stromal cells negatively regulate antigen-specific CD4+ T cell responses. J Immunol 193: 1636-44 Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, et al. 2011. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 12: 1096-104 Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, et al. 2011. Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS ONE 6: e27618 Khan O, Headley M, Gerard A, Wei W, Liu L, et al. 2011. Regulation of T cell priming by lymphoid stroma. PLoS ONE 6: e26138 Aloisi F, Pujol-Borrell R. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol 6: 205-17 Pitzalis C, Jones GW, Bombardieri M, Jones SA. 2014. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nature reviews. Immunology 14: 447-62 Carragher DM, Rangel-Moreno J, Randall TD. 2008. Ectopic lymphoid tissues and local immunity. Seminars in Immunology 20: 26-42 Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. 2012. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 33: 297-305 Link A, Hardie DL, Favre S, Britschgi MR, Adams DH, et al. 2011. Association 7 15. of T-Zone Reticular Networks and Conduits with Ectopic Lymphoid Tissues in Mice and Humans. The American Journal of Pathology 178: 1662-75 Bombardieri M, Barone F, Lucchesi D, Nayar S, van den Berg WB, et al. 2012. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol 189: 3767-76 How are you planning to ensure adequate supervision? Adequate daily supervision in the lab will be provided by Dr. Saba Nayar, a Post. Doc working with Dr. Barone that has a large experience in the TLO model and in the analysis of stromal cells subsets described in the application. Dr. Barone and Dr. Fletcher will set up regular meetings to discuss progresses and development of the project. The student role. The student will be involved in tissue dissection, digestion and processing. The student will be learning FACS analysis, use of confocal microscopy, cell sorting and PCR analysis. 8 Lead Supervisor: Dr. Francesca Barone Contact Email: Telephone: f.barone@bham.ac.uk 07726899695/ 01213713248 Co Supervisor: Dagmar Scheel-Toellner Project Title: RANK-ligand positive B cells: a new pathogenic population in autoimmunity Department: Rheumatology Research Group Will the project require a Home Office working with animals licence? Yes Is the Project Cancer related? No Project Outline Background: RANK/RANK-L interaction is critical for lymph node establishment in embryonic life. More recently this pathway has been involved in the formation of lymphoid tissue at ectopic sites, a phenomenon that often occur in chronic inflammatory conditions. Sjogren’s’ syndrome (SS) is a chronic inflammatory autoimmune disease which affects 0.1-0.4% of the UK adult population. It involves loss of function of the exocrine glands and systemic manifestations due to B cell hyperactivation. In 20-40% of patients the lymphocytes infiltrating the salivary glands accumulate to form structures resembling lymph nodes. These tertiary lymphoid organs (or TLOs) contain organized T and B cell aggregates with reticular networks of Podoplanin/gp38+ stromal cells and formation of follicular dendritic cell network. TLOs can in some cases develop into malignant Mucosal Associated Lymphoid Tissue (MALT) lymphoma. We have recently described a proinflammatory B cell population in rheumatoid arthritis that expresses RANK-L and is involved in disease pathogenesis. These cells express a unique surface protein that, in humans, is also expressed by MALT lymphoma B cells. We have recently detected these cells in glands of SS patients and we have data showing a similar proinflammatory phenotype to that observed in rheumatoid arthritis. In mice we can study this proinflammatory B cell population taking advantage of their expression of RANK-L. Project objective: In this project we use an inducible model of SS in salivary glands of wild type mice by delivery of a replication deficient adenovirus. We will investigate the presence and function of RANK-L+ B cells in relationship with the development of the TLO. Plan of Investigation: Adenovirus induced formation of TLOs in C57/BL6 SG (cannulation model). This model involves delivery of replication-defective adenovirus 5 (AdV5) via retrograde cannulation of submandibular gland excretory ducts in C57BL/6 9 mice. Within 15 days post cannulation TLO form that present T/B cell segregation and expression of lymphoid cytokines and chemokines. TLO analysis will be performed during the whole time course post cannulation (day 2,5,8,15 and 23 post cannulation) assessing by immunofluorescence (IF) follicle size, degree of T/B cell segregation and area, FDC formation and expression and distribution of lymphoid chemokines. Analysis of RANK-L+ B cells Using flow cytometry on digested tissue we will analyse the different cellular components of the aggregates both in terms of number and phenotype of DCs, T and B cells. On isolated lymphocytic populations we will assess the expression RANK-L by flow cytometry. B cells will be also sorted and PCR analysis will be performed to confirm the protein data and to investigate, in this population the expression of proinflammatory cytokines. Using confocal microscopy we will address the presence and distribution of RANK-L+ B cells within the TLO and their interaction with local immune cells. References Yeo L, Lom H, Juarez M, Snow M, Buckley CD, Filer A, Raza K, Scheel-Toellner D. Expression of FcRL4 defines a pro-inflammatory, RANKL-producing B cell subset in rheumatoid arthritis. Ann Rheum Dis. 2014 Jan 15 Bombardieri M, Barone F, Lucchesi D, Nayar S, van den Berg WB, et al. 2012. Inducible tertiary lymphoid structures, autoimmunity, and exocrine dysfunction in a novel model of salivary gland inflammation in C57BL/6 mice. J Immunol 189: 376776 Mueller SN, Germain RN. 2010. Stromal cell contributions to the homeostasis and functionality of the immune system. Nature Reviews Immunology Pitzalis C, Jones GW, Bombardieri M, Jones SA. 2014. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nature reviews. Immunology 14: 447-62 Neyt K, Perros F, GeurtsvanKessel CH, Hammad H, Lambrecht BN. 2012. Tertiary lymphoid organs in infection and autoimmunity. Trends Immunol 33: 297-305 Link A, Hardie DL, Favre S, Britschgi MR, Adams DH, et al. 2011. Association of TZone Reticular Networks and Conduits with Ectopic Lymphoid Tissues in Mice and Humans. The American Journal of Pathology 178: 1662-75 How are you planning to ensure adequate supervision? Adequate daily supervision in the lab will be provided by Ms. Joana Campos and Dr. Saba Nayar, a third year PhD student and a post doctoral fellow working with Dr. Barone. Both have a large experience in the induction and analysis of the TLO model described in the application. Dr. Barone and Dr. Sheel-Toellner will have regular meetings to discuss progresses and development of the project. The student role. 10 The student will be involved in induction of the model, tissue dissection, digestion and processing. The student will be learning FACS analysis, use of confocal microscopy, cell sorting and PCR analysis. 11 Lead Supervisor: Andrew Beggs (Cancer Sciences) Contact Email: Telephone: a.beggs@bham.ac.uk 0121 414 7458 Co Supervisor: Aditi Kadhere (School of Biosciences) Project Title: Investigation of the biological role of the long non-coding RNA OR3A4 in the development of oesophageal cancer Department: Schools of Cancer Sciences & Biosciences Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? Yes Project Outline Oesophageal adenocarcinoma (OADC) is a cancer rapidly increasing in incidence, with the UK having the highest rate in Western Europe. Typically OADC is driven by TP53 mutation however the events that initiate OADC are unclear. It appears to start in a pre-malignant lesion, known as Barretts Oesophagus (BO) however the molecular drivers that cause this to progress to OADC are unclear. We have recently carried out an epigenome wide association study that has highlighted the role of expression of the non-coding RNA OR3A4 in the progression of BO to OADC. What is unclear, however, is what biological role OR3A4 plays in carcinogenesis, given its primary role as a non-coding olfactory receptor pseudogene. We hypothese that it has a novel carcinogenic function and wish to explore its biological relationships. We aim to knock down OR3A4 in a cell line model and ascertain the pathways affected by this using gene expression studies. We will employ a new technique, individual-nucleotide resolution CLIP, to ascertain the mechanism behind its action. References 1)Kretz M et al. Control of somatic tissue differentiation by the long non-coding RNA TINCR. Nature. 493(7431): 231–235. 2) Broughton JP, Pasquinelli AE. Identifying Argonaute binding sites in C. elegans using iCLIP. Methods 63 (2013) 119–125. How are you planning to ensure adequate supervision? Regular supervisory meetings with student with both supervisors Technical support staff in both labs will carry out day to day supervision. The student role. siRNA knockdown of OR3A4 & gene expression via RT-QPCR to produce OR3A4 null cell line; Knockdown and wildtype line will then be subjected to RNA/protein interaction mapping via iCLIP technique and products RNA sequenced. 12 Lead Supervisor: Glenn Matthews (Cancer Sciences) Contact Email: Telephone: a.beggs@bham.ac.uk 0121 414 7458 Co Supervisor: Andrew Beggs (Cancer Sciences) Project Title: Validation of novel biomarkers for progression of Barrett’s oesophagus to adenocarcinoma Department: Schools of Cancer Sciences Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? Yes Project Outline Oesophageal adenocarcinoma (OADC) is a cancer rapidly increasing in incidence, with the UK having the highest rate in Western Europe. Typically OADC is driven by TP53 mutation however the events that initiate OADC are unclear. It appears to start in a pre-malignant lesion, known as Barretts Oesophagus (BO) however the molecular drivers that cause this to progress to OADC are unclear. We have recently carried out an epigenome wide association study that has highlighted multiple differentially methylated loci of significance in the transition from BO to OADC. We now wish to test a subset of these loci in a new sample set of patients with BO to see whether they continue to be significantly differentially methylated. This work will allow us to decide which markers should be taken forward into a prospective clinical trial. References 1. Dilworth MP, Beggs AD et al. A novel methylation biomarker for oesophageal adenocarcinoma. European Journal of Surgical Oncology 40 (11), S24 2. Leong KJ et al. Biomarker‐based treatment selection in early‐stage rectal cancer to promote organ preservation. British Journal of Surgery 101 (10), 1299-1309. How are you planning to ensure adequate supervision? Regular supervisory meetings with student with both supervisors Technical support staff in both labs will carry out day to day supervision. The student role. DNA extraction of validation samples, DNA quantification, pyrosequencing assay design and bisulphite pyrosequencing. Statistical analysis of results. 13 Lead Supervisor: Dr. Fedor Berditchevski Contact Email: Telephone: f.berditchevski@bham.ac.uk 0121 -414 2801 Co Supervisor: Dr.Abeer Shaaban Project Title: Histological markers of response/resistance to neoadjuvant therapy in breast cancer Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Breast cancer is the commonest cancer in females (1:8 females will develop breast cancer during their lifetime). One of the modalities of treatment is neoadjuvant chemotherapy (NACT). Some patients show complete response with no residual tumour tissue following NACT while others show partial or no response. The practice of using neoadjuvant chemotherapy has evolved over the last 10 years and now is an opportune time to review what we have learnt and re-define criteria for advising patients about this approach to breast cancer treatment. Previous reported trials do not take into account the impact of Herceptin (HC) in appropriate patients. There is limited data in the literature on factors that determine response/resistance to NACT. Tetraspanins comprise a large family of evolutionarily conserved, fourtransmembrane domain proteins. The tetraspanin proteins are known to facilitate the assembly of specialized molecular aggregates on plasma and intracellular membranes known as tetraspanin-enriched microdomains1. These microdomains also include adhesion receptors (integrins) and receptor tyrosine kinases2. Tetraspanins are thought to influence tumour migration and also tumour proliferation 3 We have previously analysed the expression of those markers in tumours of various tissues including the breast4, and have in vitro evidence of a link response to HC treatment. However, the relation between tetraspanins and response to neoadjuvant HC has not been studied before. The project aims to identify whether expression of tetraspanin proteins predicts response/resistance to neoadjuvant HC treatment by relating tumour features and molecular marker expression in pre-treatment core biopsy to tumour response. Where there is residual invasive carcinoma, these will also be analysed for expression of tetraspanins and other markers of interest. Expression profiling will be performed using Laser Capture Microdissection (LCM) and qPCR. Slides of patients who underwent neoadjuvant Herceptin treatment will be selected, paraffin blocks retrieved and sections cut. The 14 tissues will be used for extracting RNA from tumour tissues and subsequent preparation of the templates for quantitative PCR (qPCR). Where appropriate immunohistochemical staining will be performed using standard protocols. Analyses will be performed on core biopsy pre-treatment samples and posttreatment residual material where available. References 1. 2. 3. 4. 5. Hemler, 2008 Nat Rev Drug Discov, 7:747-758 Novitskaya et al., 2013 Oncogene, doi:10.1038/onc.2013.231 Romanska and Berditchevski, 2011 J Pathol, 223:4-14 Shaaban et al., 2012 Breast Cancer Res Treat, 133:949-58 Fend and Raffeld, 2000 J Clin Pathol How are you planning to ensure adequate supervision? This project involves two supervisors: Dr Berditchevski is based in the School of Cancer Sciences and he will be able to supervise the student in pPCR protocols , and, if required, in biochemical and cell biology experiments. The student will be supervised on a daily basis by experienced post-docs in Berditchevski’s laboratory. Dr.Shaaban is based in the Pathology Department, and she will supervise the student’s work involving immunohistochemical and histology techniques. Both supervisors will liaise regularly to ensure that the student is gaining adequate training and guidance to complete the project. The student role. The student will be mainly based in the School of Cancer Sciences for qPCR work where s/he will be working on breast cancer sections identified by Dr.Abeer Shaaban. All the protocols and techniques are established in the department- and the student will initially be taught these techniques followed by focussing on the specific tetraspanin molecules which have been investigated in the past in analogous studies (e.g. CD151, Tspan6). The experiments involving IHC staining will be performed at the Department of Pathology, QEHB. The student will attend lab meetings throughout their training and will be given an opportunity for a short presentation at the end of the project. 15 Lead Supervisor: Contact Email: Telephone: Roy Bicknell bicknelr@adf.bham.ac.uk 0121 414 4085 Co Supervisor: Zsuzsanna Nagy Project Title: Mechanistic study of a prostate cancer gene Department: Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Prostate cancer associated transcript 19 (PCAT19) has recently been associated with aggressive prostate cancer and increased mortality by two large (20,000 patients) independent Genome-wide association studies (GWAS) studies. One in UK (1), the other in the USA (2). PCAT19 is of particular interest to our group because we identified it as being restricted to the vasculature (3), but until now no function has been ascribed to it We have determined that the upregulation of PCAT19 blocks the progression of the cell cycle through G2/M phase and regulates the expression of CBX5 (chromobox homolog 5), a mediator of gene silencing (unpublished data). However the mechanism by which this regulation occurs is not yet known. References 1. Amin Al Olama, A. Kote-Jarai, Z. Schumacher, FR. et al. 2013. A metaanalysis of genome-wide association studies to identify prostate cancer susceptibility loci associated with aggressive and non-aggressive disease. Human Molecular Genetics. 22(2):408-15. 2. Shui, IM. Lindström, S. Kibel, AS. et al. 2014. Prostate cancer (PCa) risk variants and risk of fatal PCa in the National Cancer Institute Breast and Prostate Cancer Cohort Consortium. European Urology. 65:1069-1075. 3. Huminiecki, L. Bicknell, R. 2000. In silico cloning of novel endothelial-specific genes. Genome Research. 10(11):1796-806. How are you planning to ensure adequate supervision? One-to-one meetings at least once per month, plus fortnightly group meetings. The student role. The aims of this project are: 1. To experimentally validate the interaction between PCAT19 and CBX5 2. Modify the expression of PCAT19 and explore it's association with the cell cycle. 16 Lead Supervisor: Drs Sarah Blair-Reid and Jo Morris Contact Email: Telephone: s.a.blairreid@bham.ac.uk 44033 Co Supervisor: N/A Project Title: Generation and characterisation of cell line bearing cancer-predisposition gene changes. Understanding early-stage breast cancer. Cancer sciences Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Understanding the initiation of cancer development has historically been difficult to study in a human context, and researchers have relied on genetic manipluation of mice to study it. Now new technology of genome editing has changed this and specific genetic changes can be placed in ‘normal’ human cells in order to examine cell changes. We have developed novel normal human breast epitheial cells that have gene changes associated with breast cancer predisposition They possess changes in p53 or 53BP1. These changes can also modulate how responsive breast tumors are to treatment. You will: Aid in the generation of cell lines carrying a variety of mutations, introduced using genome editing technology (CRISPR) Establish the survival characteristics of these cells, using colony formation assays Determine whether these cells have perturbed responses to DNA damaging agents and address their ability to repair DNA using a variety of techniques Charaterisation of these cells will give us new insights into the very early faults that arise in human cells once a mutation occurs but before the cell has transformed into a cancer cell. References Hyongbum Kim and Jin-Soo Kim, A guide to genome engineering with programmable nucleases. Nature Reviews Genetics, 2014. 15: 321-334 Zimmermann, M. and T. de Lange, 53BP1: pro choice in DNA repair. Trends in cell biology, 2013. Carvajal, L.A. and J.J. Manfredi, Another fork in the road--life or death decisions by the tumour suppressor p53. Embo Reports, 2013. 14(5): p. 41421 17 How are you planning to ensure adequate supervision? The student will be supervised by post-doc Dr Sarah Blair-Reid on a day to day basis. Dr Blair-Reid has experience working with a wide variety of cell lines and is familiar with genomic editing technology and the assays to be used. Dr Morris will meet with the student weekly for a one-to-one session to discuss progress and planning of the write-up. In addition the student will present to members of the Morris Lab. The student role. The student will be expected to be in the laboratory for the majority of the time on project and to bring a professional and determined approach. The project will involve mammalian cell culture, the use of chemotherapeutic agents in tissue culture, dual colour immunofluorescence and microscopy, and colony assays. The student will be expected to do a literature search and small write-up around Christmas, to prepare and plan their laboratory work, in consultation with Drs BlairReid and Morris, to articulate their data and results at weekly meetings, keep accurate and up-to-date lab books and to prepare a 40 minute talk on their project at its beginning and end. 18 Lead Supervisor: Constanze Bonifer Co Supervisor: Justin Loke Contact Email: Telephone: lokej@bham.ac.uk 07947352222 Project Title: Identification of common and distinct epigenetic reprogramming properties of core-binding factor fusion proteins School of Cancer Sciences Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Mutations involving the transcription factor RUNX1, a member of the core binding factor (CBF) family, are one of the most frequent causes of acute myeloid leukaemia (AML) but how these molecules block differentiation is only poorly understood. RUNX1 activity can be altered as a result of translocations. This results in targeting ectopic activities to RUNX1 binding sites by fusing its DNA-binding (RUNT) domain to those of other protein domains. Currently, it is not known whether different RUNX1 translocation products bind to similar targets and deregulate similar pathways. We have recently shown that expression of the translocation RUNX1-ETO leads to the reprogramming of the epigenetic landscape, and to alterations of transcription factor binding at thousands of genomic sites (1, 2). Knock-down of RUNX1-ETO largely reverses reprogramming. We are currently studying a second RUNX1 translocation, the t(3;21), which fuses the RUNT domain to the entire EVI-1 gene, resulting in the expression of the fusion protein RUNX1-EVI-1. Using genome-wide assays, we will determine the RUNX1-EVI-1 specific cistrome and compare it to that of RUNX1-ETO. We can show a functional siRNA specifically depleting RUNX1-EVI-1 results in phenotypic changes indicative of differentiation due to changes in gene expression of key transcription factors. DNA is packaged into chromatin structures involving histones. Post translational modification of histone subunits mediate DNA processes such as transcription. We aim to identify common and distinct epigenetic reprogramming properties between the two core-binding factor fusion proteins. One histone mark that is likely to have a different distribution, between the two leukaemic subtypes, is histone 3 lysine 9 trimethylation (H3K9me3), a defining feature of transcriptionally repressive heterochromatin structure(3). EVI-1 has been shown to mono-methylate H3K9 (4) and can interact with SUV39H1 and G9a (5), enzymes which can produce H3K9me3. References 1. Ptasinska A, Assi Salam A, Martinez-Soria N, Imperato Maria R, Piper J, Cauchy P, et al. Identification of a Dynamic Core Transcriptional Network in t(8;21) AML that Regulates Differentiation Block and Self-Renewal. Cell Reports. 2014;8(6):1974-88. 2. Ptasinska A, Assi SA, Mannari D, James SR, Williamson D, Dunne J, et al. Depletion of RUNX1/ETO in t(8;21) AML cells leads to genome-wide changes in chromatin structure and transcription factor binding. Leukemia. 2012;26(8):1829-41. 3. Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21(3):381-95. Epub 2011/02/16. 19 4. Pinheiro I, Margueron R, Shukeir N, Eisold M, Fritzsch C, Richter F, et al. Prdm3 and Prdm16 are H3K9me1 Methyltransferases Required for Mammalian Heterochromatin Integrity. Cell. 2012;150(5):948-60. 5. Spensberger D, Delwel R. A novel interaction between the proto-oncogene Evi1 and histone methyltransferases, SUV39H1 and G9a. FEBS Letters. 2008;582(18):2761-7. How are you planning to ensure adequate supervision? 1) Day to day working with clinical research fellow, Justin Loke 2) Supervision meetings with Prof Constanze Bonifer This is also a dynamic and friendly lab with a number of post-doctoral researchers, PhD students and technicians, all of whom are very accessible. The student role. 1) Investigate whether RUNX1-EVI-1 can interact with SUV39H1 and G9a by coimmunoprecipitation. 2) We are currently transducing an inducible RUNX1-EVI-1 shRNA into a cell line with the t(3;21) translocation. Select clones of RUNX1-EVI-1 shRNA t(3;21) cell line. Show that the shRNA specifically targets RUNX1-EVI-1 by qPCR and Western blot 3) Investigate by ChIP whether RUNX1-EVI-1 binding sites are associated with H3K9me1/3 and whether these marks are reversible upon knock down of RUNX1EVI-1. 4) Compare patterns of H3K9me3 between RUNX1-EVI-1 and RUNX1-ETO cell lines. The student would also be expected to attend the weekly journal clubs, lab meetings and departmental talks. 20 Lead Supervisor: Dr S. John Curnow Contact Email: Telephone: s.j.curnow@bham.ac.uk Co Supervisor: Dr Mike Douglas Project Title: Repertoire and phenotype of T and B cells in patients with early multiple sclerosis Department: Immunity & Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Although the central nervous system (CNS) is considered to be immune privileged there is a distinct population of CCR7+ central memory T cells present in the cerebrospinal fluid (CSF) in healthy individuals. When inflammation occurs (e.g. in multiple sclerosis) there is an increase in the number of T cells found in the CSF. However, the most distinct difference is the appearance of antibody-secreting B cells and consequent presence of antibody in the CSF. There is also excess free light chain which provides a sensitive and specific test to support the diagnosis of MS. Research within the group is currently addressing the repertoire and phenotype of the T and B cells in patients with early MS. The project would align to these research aims with the specifics depending on the progress of the ongoing project. Cells will be analysed using flow cytometry and real-time PCR for a range of molecules involved in the selection of the T/B cell repertoire. Samples (CSF and blood) from patients with early disease (clinically isolated syndrome) will be compared to those who have already experienced a relapse of disease (relapsing-remitting MS). Additional blood samples at follow-up will also be analysed. References Hassan-Smith G. High sensitivity and specificity of elevated cerebrospinal fluid kappa free light chains in suspected multiple sclerosis. 2014 J. NeuroImmunol 276:175– 179. Vafaii, P. Detection of B-cell populations with monotypic light chain expression in cerebrospinal fluid specimens from patients with multiple sclerosis by polychromatic flow cytometry. Cytometry B Clin Cytom, 2014. 86(2): p. 106-10. Mullen KM. Expression of CCR7 and CD45RA in CD4+ and CD8+ subsets in cerebrospinal fluid of 134 patients with inflammatory and non-inflammatory neurological diseases. 2012 J Neuroimmunol 249:86-92. How are you planning to ensure adequate supervision? The student will be trained in the lab by Dr Curnow’s technician and receive additional support from other members of the group. Dr Curnow holds (at least) weekly meetings with each student and the group meets once a week to discuss data, future plans and the scientific literature. The labs at the Centre for Translational Inflammation Research in the QE Hospital and fully equipped and provide an excellent environment for the research. The student role. 21 The student will prepare CSF and peripheral blood samples from patients with MS as well as disease and healthy controls. They will analyse expression using flow cytometry and real-time PCR. Data will be collated with any clinical information. 22 Lead Supervisor: Dr Nick Davies Contact Email: Telephone: n.j.davies@bham.ac.uk ext 46823 Co Supervisor: Prof T Stankovic Project Title: Assessment of potential of ATR inhibitor as a novel therapeutic for treatment of diffuse large B cell lymphoma Cancer Sciences Department: Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? Yes Project Outline Diffuse large B cell lymphoma (DLBCL) is the most common tumour of lymphoid tissues.1 One of the characteristics of DLBCL is impaired DNA damage responses (DDR) as a result of inactivation or loss of p53 expression due to overexpression of transcriptional repressor BCL6.2-4 Furthermore, DLBCL with p53 mutations show a particularly poor prognosis.5, 6 Ataxia Telangiectasia Related (ATR) is a regulator of the cell cycle and a key mediator of cellular responses to replication stress and DNA damage.7 Recently, we have demonstrated that chronic lymphocytic leukaemia tumours with p53 inactivation are sensitive to inhibition of ATR due to accumulation of intolerable levels of DNA double strand breaks (DSBs).8 Furthermore we have observed that ATR inhibition synergises with conventional chemotherapies to improve cell killing. Our hypothesis is that a subset of DLBCL tumours will be sensitive to ATR inhibition due to the loss of their p53 function. To test this hypothesis a cohort of DLBCL cell lines will be screened for TP53 mutations, ATR, p53 and BCL6 expression as well as for DDR. A selection of these cell lines with and without a clear p53 defect will then be used to assess efficacy of ATR inhibition in vitro, both as a single agent and in combination with conventional chemotherapies as well as BCL6 inhibitors. We anticipate that inhibition of ATR will provide a novel therapeutic target in DLBCL, particularly in combination with other agents due to its reduced DDR response. References 23 1. 2. 3. 4. 5. 6. 7. 8. Carbone A, Gloghini A, Kwong YL, Younes A. Diffuse large B cell lymphoma: using pathologic and molecular biomarkers to define subgroups for novel therapy. Ann Hematol 2014;93(8):1263-77. Young KH, Leroy K, Moller MB, Colleoni GW, Sanchez-Beato M, Kerbauy FR, et al. Structural profiles of TP53 gene mutations predict clinical outcome in diffuse large B-cell lymphoma: an international collaborative study. Blood 2008;112(8):3088-98. Phan RT, Saito M, Basso K, Niu H, Dalla-Favera R. BCL6 interacts with the transcription factor Miz-1 to suppress the cyclin-dependent kinase inhibitor p21 and cell cycle arrest in germinal center B cells. Nat Immunol 2005;6(10):1054-60. Jardin F, Ruminy P, Bastard C, Tilly H. The BCL6 proto-oncogene: a leading role during germinal center development and lymphomagenesis. Pathol Biol (Paris) 2007;55(1):73-83. Winter JN. Prognostic markers in diffuse large B-cell lymphoma: Keys to the underlying biology. Curr Hematol Malig Rep 2007;2(4):235-41. Ichikawa A, Kinoshita T, Watanabe T, Kato H, Nagai H, Tsushita K, et al. Mutations of the p53 gene as a prognostic factor in aggressive Bcell lymphoma. N Engl J Med 1997;337(8):529-34. Fokas E, Prevo R, Hammond EM, Brunner TB, McKenna WG, Muschel RJ. Targeting ATR in DNA damage response and cancer therapeutics. Cancer Treat Rev 2014;40(1):109-17. Kwok M, Davies N, Agathanggelou A, Smith E, Yates E, Brown J, et al. ATR inhibition exacerbates replication stress in TP53 or ATM deficient CLL cells and enhances sensitivity to chemotherapy and targeted therapy. Manuscript in preparation. How are you planning to ensure adequate supervision? I am a senior post-doc in Professor Stankovic’s group and am regularly present in the lab and will supervise/ train the student on a daily basis until the student feels comfortable with the techniques. We will have regular meetings to discuss results, further experiments and the direction that the project might take. Furthermore, the Stankovic group has weekly meetings where work is presented and ongoing projects are discussed, providing an additional platform for the training. The student role. The student will be expected to show an enthusiastic approach to the project. The student will learn a number of techniques and hopefully enjoy his/her time in the lab. At the later stages the student will be expected to present the data at the Stankovic group meeting as well as have some input in the experimental design and decisions on the direction that the project might take. 24 Lead Supervisor: Dr Jo Morris Contact Email: Telephone: j.morris.3@bham.ac.uk or r.m.densham@bham.ac.uk x4143016 Co Supervisor: Dr Ruth Densham Project Title: Characterising patient variants of the Breast and ovarian cancer predisposition gene, BRCA1. Department: School of Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline An increased risk of breast and ovarian cancer is associated with inheritance of a faulty copy of the Breast Cancer Susceptibility Gene 1 (BRCA1). While BRCA1 has many roles in the cell, the only intrinsic enzymatic activity of the protein is found within the highly conserved N-terminus. Clinically relevant missense variants found within this region suggest a role for this activity in tumour suppression1. Our recent unpublished data has identified a novel regulatory region in BRCA1 that enhances the enzymatic activity of the protein. We have also shown that patient variants that alter the protien in this new region also reduce the enzymatic activity of BRCA1. These exciting new results suggest that the enzyme activity of BRCA1 may be important to its ability to suppress cancer development. Now we want to characterise whether these patient variants change the cellular function of BRCA1, and, ultimately, relate to tumour development. Using cutting edge genome editing techniques2 that we have optimised in our lab, the student will generate a series of BRCA1 patient missense variants within a model human cell line. We will then characterise the role of these variants in the response to chemotherapy treatments and in tumour progression using cell survival assays, fluorescent microscopy and standard lab techniques. References 1. Morris JR et al. Genetic analysis of BRCA1 ubiquitin ligase activity and its relationship to breast cancer susceptibility. Hum Mol Genet. (2006) 15(4):599-606. 2. Kim H, Kim JS. A guide to genome engineering with programmable nucleases. Nat Rev Genet. (2014) 15(5):321-34. How are you planning to ensure adequate supervision? Our lab is experienced in mentoring students. There will be weekly 1-2-1 meetings with the Group Leader and hands-on lab training from experienced post-docs. The student role. The student will learn: CRISPR genome editing, tissue culture, survival assays, DNA damage response assays, basic microscopy, western blotting, PCR. In addition, the student will be expected to attend and participate in weekly lab meetings, general lab rotas, and maintain a clear record of all experiments. 25 Lead Supervisor: Dr. Anne Fletcher Contact Email: Telephone: A.Fletcher@bham.ac.uk Collaborator: Dr. Mark Cobbold, Dr. Konstantin Knoblich Project Title: Lymph node stromal cells create a metastasis friendly microenvironment Department: Immunity and Infection Will the project require a Home Office working with animals licence? Yes Is the Project Cancer related? Yes Project Outline Lymph nodes (LNs) are sites where immune responses are robustly initiated, and a prevailing scientific paradigm states that lymph nodes are hostile environments for tumour cells, due to the risk of immunogenicity (1). However, for many cancers, LNs are the first observable site for metastasis, and decades of clinical evidence would suggest tumour propagation is in fact well-tolerated within LNs (2). Recent evidence suggests LNs are environments where fibroblastic reticular cells (FRCs) prevent newly activated T cells from acquiring effector functions, regardless of specificity (3-5). FRCs also express self-antigens native to various tissues, then delete specific reactive T cell clones (6-9). The relevance of these normal tolerogenic functions to anti-tumour immunity has not been assessed. This project tests the hypothesis that FRCs systematically shut down CD8+ T cell responses against tumour cells that reach the LN. Preliminary data shows that activated anti-tumour CD8+ T cells do not respond normally to tumour cells in the presence of FRCs. Objectives: 1. To test how FRCs impair T cell rejection of tumour cells in vitro, using sophisticated in vitro co-cultures and both mouse (melanoma) and human (colorectal carcinoma) experimental systems with mouse and human anti-tumour T cells. 2. To examine the fate of anti-tumour T cells after being activated in the presence of tumour cells and FRCs. We will explore whether T cells are temporarily or permanently silenced, and whether the suppression can be overcome. 3. To test the effect of FRCs on tumour cell killing, using in vitro and in vivo systems. We will utilise europium release assays and transgenic mice that permit deletion of FRCs. Techniques: The student will be taught relevant cell culture techniques, flow cytometry, confocal microscopy, and skills required to study in vivo cancer models. Outcomes: We are hopeful (but cannot guarantee for obvious reasons!) that the work produced by the student will be used in presentations and ideally submitted for publication when a body of work comes together. References and further reading 26 1. 2. 3. 4. 5. 6. 7. 8. 9. O. Preynat-Seauve, E. Contassot, P. Schuler, V. Piguet, L. E. French, B. Huard, Extralymphatic tumors prepare draining lymph nodes to invasion via a T-cell cross-tolerance process. Cancer Res 67, 5009-5016 (2007). S. A. Stacker, M. G. Achen, L. Jussila, M. E. Baldwin, K. Alitalo, Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2, 573-583 (2002). O. Khan, M. Headley, A. Gerard, W. Wei, L. Liu, M. F. Krummel, Regulation of T cell priming by lymphoid stroma. PLoS One 6, e26138 (2011). V. Lukacs-Kornek, D. Malhotra, A. L. Fletcher, S. E. Acton, K. G. Elpek, P. Tayalia, A. R. Collier, S. J. Turley, Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol 12, 1096-1104 (2011). S. Siegert, H. Y. Huang, C. Y. Yang, L. Scarpellino, L. Carrie, S. Essex, P. J. Nelson, M. Heikenwalder, H. Acha-Orbea, C. D. Buckley, B. J. Marsland, D. Zehn, S. A. Luther, Fibroblastic reticular cells from lymph nodes attenuate T cell expansion by producing nitric oxide. PLoS One 6, e27618 (2011). A. L. Fletcher, V. Lukacs-Kornek, E. D. Reynoso, S. E. Pinner, A. Bellemare-Pelletier, M. S. Curry, A. R. Collier, R. L. Boyd, S. J. Turley, Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med 207, 689697 (2010). A. L. Fletcher, D. Malhotra, S. J. Turley, Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol 32, 12-18 (2011). D. Malhotra*, A. L. Fletcher*, J. Astarita, V. Lukacs-Kornek, P. Tayalia, S. F. Gonzalez, K. G. Elpek, S. K. Chang, K. Knoblich, M. E. Hemler, M. B. Brenner, M. C. Carroll, D. J. Mooney, S. J. Turley, Transcriptional profiling of stroma from inflamed and resting lymph nodes defines immunological hallmarks. Nat Immunol 13, 499-510 (2012). A. L. Fletcher, J. S. Elman, J. Astarita, R. Murray, N. Saeidi, J. D'Rozario, K. Knoblich, F. D. Brown, F. A. Schildberg, J. M. Nieves, T. S. Heng, R. L. Boyd, S. J. Turley, B. Parekkadan, Lymph node fibroblastic reticular cell transplants show robust therapeutic efficacy in high-mortality murine sepsis. Sci Transl Med 6, 249ra109 (2014). How are you planning to ensure adequate supervision? The student and I will have formal weekly meetings, but as I am often in the lab myself, there will be many informal chances to discuss data, methods, progress, science, and anything else that comes up. The student will also present regularly to our small group for feedback on results, as well as getting practice at presenting scientific findings. I have supervised students for this length of project several times before, and understand that close supervision and hands-on practical training is required in the beginning, but that the student may wish to explore a little more scientific independence by the end of the project. However, no one supervisory style fits all and for this type of project I try to work with the student in a way tailored to their strengths and challenges. The student role. The student’s role is to work through a series of experiments, and learn step by step how to execute certain important assays. Some assays may require a few practices before they are mastered, and some planned experiments may change depending on previous results obtained. In this case, I would work with the student’s input to decide what experiment to do next. The student should be able to interpret results, think critically about what they mean with reference to literature, and present and discuss data for friendly peer review within the laboratory. 27 Lead Supervisor: Dr Aga Gambus Contact Email: Telephone: a.gambus@bham.ac.uk 01214149237 Co Supervisor: Project Title: How is the replisome disassembled at the end of replication once it has done its job? Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline We all have about 2m of DNA in every one of our cells. Every time cells in our body want to divide they need to first duplicate their genome - their DNA. Ever since the discovery od DNA structure scientists are trying to describe how this process of DNA replication works in detail, as its perfect execution is essential to maintain genomic stability and prevent cancer formation. We know that one of the main causes of mutations that lead to cancer development are unrepaired mistakes that arise during the process of duplication. Thus, a lot of work had been done towards understanding the synthesis of DNA and the initiation stage of DNA replication. However, very little is known as to how this process finishes, which is the subject of our paper. Our recent work has shown a first glimpse of the mechanism that unloads the replication machinery at the end of replication. The replication machinery (replisome) is formed of about 150 proteins and built around a few key organising centers. One of them is a replicative helicase, which is the protein complex that can unwind double stranded DNA to open it to create the templates for DNA synthesis. The helicases are loaded onto DNA in hundreds of thousands, and about 30-50 thousand of them are activated per cell during DNA replication. They then go through DNA, unwinding it, until they meet a helicase coming from the opposite direction. These helicases, once working, are very precious as if they get taken off DNA by mistake they cannot be put back on. This means however that at the end, when they meet each other they have to be taken off by some sophisticated mechanism. We have shown in our work that one of the components of this helicase (Mcm7 subunit) is modified at the final stage of replication by attachment of a small protein modifier called ubiquitin. This modification allows it to be recognised by a protein remodeler, which takes it off DNA allowing the whole process to complete. As this is the first time we start to learn about this stage of replication there is of course a lot more work that needs to be done to characterise it in more detail and to learn the identity of all the essential factors needed for its 28 execution. The proposed project aims to understand better how the modified Mcm7 (and the rest of the replisome) is taken off DNA by the protein remodeler p97 and the fate of the removed helicase. To conduct this research, the Student will use a cell-free system that recapitulates a whole round of DNA replication in vitro and thus is invaluable for biochemical studies of eukaryotic DNA replication and DNA repair processes. References Polyubiquitylation drives replisome disassembly at the termination of DNA replication. Moreno SP, Bailey R, Campion N, Herron S, Gambus A. Science. 2014 Oct 24;346(6208):477-81. doi: 10.1126/science.1253585. PMID: 25342805 [PubMed - in process] I will supervise the Student myself on a day to day basis and teach the student all the techniques required. I will make sure that the Student understands the project in depth, that her / his lab book is kept up to date and that we discuss the progress of the project on the regular basis. Finally, I plan to ask the student to prepare a number of presentations about different aspects of the project to ensure that he / she gathers all required literature background knowledge over the duration of the project rather than leaving it till the end.. The student role. I expect the Student to become proficient in all the techniques he / she will need to use and to be able to carry on the experiments by him / herself after the initial training. All of the work carried out by the Student for the purpose of this project is laboratory based (wet science). All of the research conducted by the Student will be novel and should result in creating data that will be used for future grant applications and publications. The Student will be a co-author of any publication resulting from this project 29 Lead Supervisor: Paloma Garcia Contact Email: Telephone: p.garcia@bham.ac.uk Co Supervisor: Laila Cancian Project Title: DNA damage studies in Haemopoietic stem cells (HSC) and progenitor cells with Mybl2 haploinsufficiency Department: Immunity and Infection, Institute of Biomedical Research Will the project require a Home Office working with animals licence? Yes (module 1) Is the Project Cancer related? YES Project Outline Many blood disorders are associated with aging. More than 62% of people diagnosed with myelodysplastic syndrome (MDS), myeloproliferative neoplasms (MPN) and leukemia are over 60 years old. It has been proposed that DNA mutations accumulate during the life of an individual as a consequence of failure to correct errors introduced in the genome during the cell replicative process, leading to either activation of oncogenes or silencing of tumor suppressors that operate as driving factors in the initiation of the disease (1,2). Deletion of the long arm of chromosome 20 is observed in a 5-10% of patients suffering haematological disorders related to ageing (MDS, MPN and leukaemias). The minimum common deleted region published so far contains 9 genes (3). One of these genes is the transcription factor MYBL2 (B-Myb) (3). We generated a mouse model that expresses half the normal dose of MYBL2, ( MYBL2 ) paralleling MYBL2 haploinsufficiency in human del20q situation (4). We have demonstrated that during ageing, mimicking what happens in humans, these mice developed haematological disorders: 50% MPN and 50% MDS disorders (5). Our hypothesis is that MYBL2 role in the maintenance of genome integrity is compromised during ageing leading to the accumulation and selection of somatic mutations which drive the development of blood disorders such as MDS and MPNs. In this project we would like to get some insight into the molecular mechanism by which MYBL2 haploinsufficiency leads to an increase in somatic mutations. Specifically, we will study the DNA repair mechanisms in MYBL2 cells (HSCs and progenitor cells). This project will imply to work with mouse models and allow the student to learn the isolation of bone marrow cells, flow cytometry, confocal microscopy, tissue culture, proliferation assays and molecular biology techniques such as western blot and Taqman quantitative PCR. References 1.-Kenyon J ,Gerson SL. 2007. The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res 35: 7557-65. 2.- Maslov AY ,Vijg J. 2009. Genome instability, cancer and aging. Biochim Biophys Acta 1790: 963-9 3.- Bench AJ, Nacheva EP, Hood TL, Holden JL, French L, Swanton S, Champion KM, Li J, Whittaker P, Stavrides G et al: Chromosome 20 deletions in myeloid malignancies: reduction of the common deleted region, generation of a PAC/BAC contig and identification of candidate genes. UK 30 Cancer Cytogenetics Group (UKCCG). Oncogene 2000, 19(34):3902-3913. 4.- García, P., Berlanga, O., Watson, R., and Frampton J. (2005) Generation of a onditional allele of the B-Myb gene. Genesis, 43: 189-95. 5.- Clarke, M.L., Dumon, S., Ward, C., Jager, R., Freeman, S., Dawood, B., Sheriff, L., Lorvellec, M., Kralovics, R., Frampton, J and García, P. MybL2 haploinsufficiency increases susceptibility to age-related haemopoietic neoplasia. Leukaemia, 2013, 27: 661–670. 6- Mohrin, M., Bourke E, Alexander D, Warr MR, Barry-Holson K, Le Beau MM, Morrison CG, Passegué E. Hematopoietic stem cell quiescence promotes error-prone DNA repair and mutagenesis. Cell Stem Cell. (2010) 6;7(2):174-85. How are you planning to ensure adequate supervision? Dr Laila Cancian will be the daily supervisor. Every time that student requires further supervision or Dr Cancian is not available, Dr Garcia will be supervising. Dr Garcia’s lab is shared with Prof Frampton, and together held around 8 postdoctoral researchers who will be also able to help and provide the right environment for the student to learn. The student will also attend regular group meetings (every 15 days) on a round table format to discuss progress/problems and to come up with solutions/ alternative approaches. The student will also benefit from the expertise of other researchers within the field by attending group meetings with DNA repair groups (fortnightly) and floor meetings with genome biology groups (weekly). The student role. The student will be studying whether haemopoietic stem cells and progenitor cells expressing half the normal dose of Mybl2 (also known as B-Myb) have a greater susceptibility to ionising radiation or other DNA damaging agents, compared to wildtype cells, in terms of cell proliferation/differentiation and ability of the cell to repair the DNA lesions. The student will then investigate the molecular mechanisms responsible for this addressing the following questions: are specific DNA damage repair pathways defective in Mybl2 haploinsufficient cells? Is Mybl2 integral part of the cell DNA damage and repair machinery? Is Mybl2 regulating the expression of DNA damage response genes or of cell proliferation/apoptosis genes? The student will perform experiments, collect data, analyse it and present it. The student will keep good record of the experimental data in his/her laboratory journal and will also participate in the daily house-keeping of the lab. The student will attend lab meetings and present and discuss his/her own work. 31 Lead Supervisor: Contact Email: Telephone: Roger Grand r.j.a.grand@bham.ac.uk 0121 414 2805 Co Supervisor: Grant Stewart Project Title: Characterisation of novel DNA damage response proteins Cancer Sciences Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline The cellular genome is subject to continuous insult resulting in the occurrence of tens of thousands of lesions in the DNA per cell per day. To counteract this, a complex series of pathways, collectively known as the DNA damage response (DDR), has evolved and this is able to detect and, if possible, repair the DNA. The inability to correct this damage is a major underlying cause of cancers as well as other diseases. These repair pathways are based on the activities of three related kinases-Ataxia Telangiectasia mutated (ATM), ATM and Rad3-related (ATR) and DNA dependent protein kinase (DNA-PK). Following detection of DNA damage, one or more of the kinases are activated leading to phosphorylation of multiple downstream targets and repair of the lesion if the damage is not too great. The DDR pathways comprise a large number of components, with, simplistically, specific proteins involved in homologous recombination, non-homologous end joining and single strand break repair amongst others. We have recently identified an important novel DDR protein, termed DIAD (Degraded and Induced by ADenovirus), which is involved in the response to replicative stress and base modifications but not double strand breaks. We have also shown by mass spectrometry that DIAD binds to the CNOT complex which has transcriptional regulatory and deadenylation properties and has been well characterized in yeast but to a much lesser extent in mammals. The aim of this project is to characterize this association in more detail and determine to what extent DIAD is unique in its DDR role or whether the CNOT complex plays a significant part in the DDR. The student will determine: 1, whether knock down of CNOT proteins (using siRNAs) has the same deleterious effect on the DDR as loss of DIAD; 2, whether DIAD and CNOT proteins locate to sites of DNA repair and 3, the role of CNOT proteins in cell cycle arrest following DNA damage. Techniques include cell culture, immunofluorescence microscopy and biochemical methods such as gel electrophoresis and western blotting. References 1.The causes and consequences of genetic heterogeneity in cancer evolution.Burrell RA, McGranahan N, Bartek J, Swanton C. Nature. 2013; 501:338-45. 2.More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance.Lukas J, Lukas C, Bartek J. Nat Cell Biol. 2011; 13:1161-9. 3.The DNA-damage response in human biology and disease. Jackson SP, Bartek J.Nature. 2009; 461:1071-8. 4.. The Ccr4--not complex. Collart MA, Panasenko OO. Gene. 2012 ; 492: 42-53 How are you planning to ensure adequate supervision? I spend most of my time in the lab and so will be able to supervise the student personally. Also other group members will be working in the lab and so will be able to help in supervision. The student role. 32 The student will be expected to carry out the experiments outlined above. Towards the end of the project it is hoped that they will contribute ideas of alternative/additional approaches. 33 Lead Supervisor: Dr Melissa Grant Contact Email: Telephone: m.m.grant@bham.ac.uk 01214665520 or 01214142652 Co Supervisor: Dr Gosia Wiench & Prof Iain Chapple Project Title: What is the role of epigenetic modifications in the decrease of glutathione in periodontitis? Department: School of Dentistry Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Glutathione (GSH) is a major antioxidant involved in maintaining redox balance. Previously we have measured glutathione in gingival crevicular fluid (GCF), a serum exudate and tissue transudate flowing from between the teeth and adjacent gingival tissue (gums). In periodontitis, severe gingival inflammation initiated by the plaque biofilm and exacerbated by an aberrant inflammatory and oxidative stress response, GCF GSH is decreased compared to healthy donors. Remarkably upon successful periodontal therapy, which reduces clinical measures of inflammation, the GSH level in GCF does not rebound to healthy levels (Grant et al 2010). We would like to understand if there are epigenetic mechanisms involved in this suppression. In periodontal disease a number of studies have been published recently investigating epigenetic modifications of inflammatory genes (eg TNFA (Zhang et al 2013); IFNG (Zhang et al 2010); PTGS2 (Zhang et al 2010); hBD2 and CCL20 (Yin & Chung 2011), indicating this is an area of research expanding rapidly. GSH is produced by a series of enzymatic steps which are controlled by the production of the rate limiting enzyme glutamate cysteine ligase (GCL). This enzyme is under transcriptional control by transcription factor Nrf2, which binds to the antioxidant response element (ARE). We have already established in vitro (H400 oral epithelial cell line) that we can induce GSH synthesis with Nrf2 agonist curcumin. Additionally we have shown that this agonist inhibits HDAC activity and can inhibit bacterial activation of the NFkB pathway (unpublished). Histone modification is a multifaceted phenomenon controlling gene expression. Mono-, di- and tri-methylation at lysine (K) residues has been associated with transcriptional silencing and activation depending on which amino acid residue is involved. Recent evidence has shown under oxidative stress conditions Histone3 lysine 4 dimethylation (H3K4me2) at the GCL gene (gclc) was increased and H3K4me3 and H3K4me1 were decreased, but these remained in place after alleviation of oxidative stress (Mishra et al 2014). Another study has shown the increases of H4R3me2 and H2R17me2 in oxidative stress induced ARE activation (Huang et al 2013). In this project we propose to use chromatin precipitation (ChIP) experiments to further our understanding of the control of GSH production in periodontitis. Initially our cell culture model, using oral epithelial cell line H400, will be used to refine skills 34 and techniques in ChIP and to explore the dynamics of gclc control under model conditions, using periodontopathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum, and induction of Nrf2 with known agonists such as curcumin, with which we have copious experience. Later studies will utilise patient tissue and potentially ChIP-Seq experiments for non-presumptive analysis of wider Nrf2 targets. References Grant et al JOURNAL OR CLINICAL PERIODONTOLOGY 2010 37:17-23 Huang et al FASEB J 2013 27: 3763-74 Mishra et al FREE RADICAL BIOLOGY AND MEDICINE 2014 75:129-39 Yin & Chung Mucosal Immunity 2011 4: 409-19 Zhang et al J Periodontol 2013 84: 1606-16 Zhang et al J Clin Perio 2010 37:953-61 Zhang et al J Dent Res 2010 89:133-7 How are you planning to ensure adequate supervision? We will plan weekly structured meetings with the student and additionally will offer an open door policy for discussion of progress. Our laboratories have many active PhD students (7) and technicians (5) whom the student can ask initial questions dayto-day. The student role. The student will be responsible for cell culture and carrying out of experiments. We will plan experiments together and discuss results prior to furthering the project. They will develop ChIP techniques under supervisory guidance; explore the back ground to the project through the literature; and will produce all documents required for completion and submission of the project. 35 Lead Supervisor: Dr. Paul Harrison Contact Email: Telephone: p.harrison.1@bham.ac.uk Co Supervisor: Professor Steve Watson Project Title: Measurement of circulating preplatelets in health and disease Department: Immunity & Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline The terminal differentiation mechanism of thrombopoiesis (platelet formation) has recently been elucidated. A new population of large platelet-like structures (i.e. preplatelets)undergoes final maturation into classical platelets within the circulating blood via a remarkable division process involving barbell formation unique to this anucleated cell. Measurement of the percentage and absolute levels of these precursors may therefore not only provide an accurate estimation of platelet turnover but provide mechanistic insights into the pathology of macrothrombocytopenia where the terminal differentiation process may also be impaired resulting in the production of large platelets. Although measurement of immature platelets by flow cytometry is an established clinical tool the assays are poorly standardized and tend to be utilised within specialised research laboratories. Routine measurement of immature platelets is also possible by using an automated full blood counter that measures the IPF (immature platelet fraction) using fluorescent flow cytometry principles. However, although this method has been shown to have clinical utility it is still unclear whether the measurement is an accurate reflection of the true circulating level of immature platelets. A significant obstacle in these assays has been the widespread use of EDTA anticoagulated whole blood which is the standard blood sample used for full blood counters and IPF measurement. Indeed it has been recently demonstrated that EDTA causes sphering of the newly identified barbell intermediates and therefore prevents their accurate identification on blood films or by full blood counters. In this project we therefore propose to set up and evaluate a number of new methods for accurately quantitating the number of these newly identified platelet intermediates in normal and pathological blood samples using alternative anticoagulants to EDTA to inhibit sphering. A comparison of various anticoagulants will be undertaken and immature platelets measured using fluorescent flow cytometry, Imagestream flow cytometry (to facilitate imaging), Sysmex Xn full blood counting (IPF measurement as a reference method) and image analysis of platelets in whiole blood or platelet rich plasma (on blood films or poly-lysine coated coverslides that have been fluorescently labelled using specific anti-platelet antibodies (e.g. anti-CD41 or CD61). To facilitate the clear identification of the intermediate preplatelets samples will also be fluorescently labelled for -tubulin. The percentage and absolute counts of the preplatelets will be established in normal samples and then applied to the study of platelet kinetics in a variety of clinical situations where platelet production is either impaired or increased. Samples from patients with various forms of macrothrombocytopenia will also be studied to determine whether there is a failure 36 in terminal maturation in some of these defects. We anticipate that this study will result in the development of a new method of accurate immature platelet enumeration with obvious clinical potential. References 1) M.S.C. Robinson, I.J. Mackie, K. Khair, R. Liesner, A.H. Goodall, G.F. Savidge, S.J. Machin, P. Harrison (1998) Flow cytometric analysis of reticulated platelets: evidence for a large proportion of non-specific labelling of dense granules by fluorescent dyes. British Journal of Haematology, 100, 351-357 2) JN. Thon, H. Macleod, AJ Begonja, J Zhu, K.C. Chen, A.Mogilner, J.H.Hartwig, J.E. Italiano (2012) Microtubule and cortical forces determine platelet size during vascular platelet production. Nature Communications, 138, 1-9. How are you planning to ensure adequate supervision? Paul Harrison will supervise the student on a day to day basis as he is an established expert on platelet counting, immature platelets and flow cytometry. The Sysmex Xn analyser is located in his laboratory. Regular personal and laboratory meetings will also be held with Steve Watson and the Birmingham platelet group to discuss project progress and for the student to present his results to a critical audience. Fluorescent microscopy and image analysis will be performed in collaboration with Steve Thomas in the same group who is an expert on high resolution fluorescent microscopy. The student role. The student will be fully trained how to perform the repertoire of techniques required for the success of the project including :- 1) preparation and processing of blood samples (2) fluorescent labelling of platelets (3) Blood and PRP film preparation on slides (4) Operation of the Sysmex Xn full blood counter (5) Flow cytometry using a variety of instruments and the ImageStream system (6) Fluorescent microscopy and image analysis. The student will record all results with a laboratory book and regularly present the data to the supervisors. The project thesis write up will be monitored so that this is deliverable by the completion date. The student will be encouraged to present and write up his work in the form of seminars and abstracts/papers. 37 Lead Supervisor: Dr. Maarten Hoogenkamp Contact Email: Telephone: m.hoogenkamp@bham.ac.uk Co Supervisor: Dr. Vesna Stanulovic Project Title: Determining the effect of LMO2 overexpression within the myeloid lineage Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline LMO2 is a transcriptional regulator that is an essential partner of several transcription factors and together they have a fundamental role in haematopoietic, vascular and cardiac development. Overexpression of LMO2 in T-cell progenitors has been shown to cause T-cell acute lymphoblastic leukaemia. A significant proportion of acute myeloid leukaemia (AML) has LMO2 overexpression but its contribution to the disease state is not known. We have generated LMO2 knockdown and overexpression models in mouse embryonic stem cells and in a myeloid progenitor cell line that upon induction differentiates into macrophages. Impaired LMO2 expression in myeloid progenitors generated large cells with an endomytotic phenotype, implying LMO2 function in chromosomal segregation. This project will start by measuring LMO2 expression before and after the knockdown induction at the mRNA (qPCR) and protein (Western blotting) level, and assessing the cellular morphology and LMO2 localisation by confocal microscopy. We will then establish the effects of high LMO2 expression, as observed in AML, on macrophage differentiation by monitoring morphology, detection of surface markers (flow cytometry), and measuring the expression of genes (qPCR) known to be involved in macrophage development. The same experiments will be performed after abolishing LMO2, mimicking potential LMO2-directed treatment. References Hoogenkamp, M. et al. (2009) Blood 114, 299-309 Matthews, J.M. et al. (2013) Nat. Rev. Cancer 13, 111-122 How are you planning to ensure adequate supervision? Both listed supervisors spend a significant proportion of their time at the bench in the laboratory, are skilled in the techniques mentioned above, and 38 have prior experience in supervision of staff and students. This ensures that supervision and support is available on a daily basis throughout the eight months project. Every technique will initially be performed together with a supervisor, hopefully resulting in more independence later into the project. In addition there will be a weekly meeting to overview and discuss the progress and next steps, or problems that have been encountered. A weekly journal club will be held in which current literature on this and related topics will be discussed. The student role. The student is expected to be motivated and to actively participate in the research group. The student will be primarily responsible for performing the experiments and interpreting the results of the above project, albeit in close association with the supervisors. The student should be motivated to learn the different techniques, which are established protocols in the lab. We will work towards the student becoming more independent over the eight months in both the practical aspects of the project as well as in the ability to interpret the obtained data. 39 Lead Supervisor: Dr. Maarten Hoogenkamp Contact Email: Telephone: m.hoogenkamp@bham.ac.uk Co Supervisor: Dr. Vesna Stanulovic Project Title: Identification of DNA binding partners of LMO proteins in the myeloid lineage Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline The four highly homologous LIM only (LMO1-4) proteins are all implicated in cancerogenesis. They are part of DNA binding transcription factor complexes, although they do not bind the DNA themselves. LMO2 and LMO4 are expressed in the myeloid lineage of the blood and LMO2 is found to be overexpressed in a large proportion of acute myeloid leukaemia (AML). Both LMO2 and LMO4 need to bind to their protein partner LIM domain binding 1 (Ldb1) for their function within the nucleus. The questions we want to address within this project are whether LMO2 and LMO4 each form distinct DNA binding complexes and whether they need to compete for Ldb1 to do this. To answer these questions we will perform antibody pull-down assays, followed by Western blotting and mass spectrometry. We will initially perform these using antibodies recognising LMO2, LMO4, and Ldb1, which will give us insight in the overlap between these complexes and which DNA binding components are present. The information from these experiments will be used to perform further pull-down assays for the newly identified proteins and chromatin immunoprecipitation to identify where the proteins bind the DNA and which genes they regulate. References Meier, N. et al. (2006) Development 133, 4913-4923 Matthews, J.M. et al. (2013) Nat. Rev. Cancer 13, 111-122 How are you planning to ensure adequate supervision? Both listed supervisors spend a significant proportion of their time at the bench in the laboratory, are skilled in the techniques mentioned above, and have prior experience in supervision of staff and students. This ensures that supervision and support is available on a daily basis throughout the eight months project. Every technique will initially be performed together with a supervisor, hopefully resulting in more independence later into the project. In addition there will be a weekly meeting to overview and discuss the 40 progress and next steps, or problems that have been encountered. A weekly journal club will be held in which current literature on this and related topics will be discussed. The student role. The student is expected to be motivated and to actively participate in the research group. The student will be primarily responsible for performing the experiments and interpreting the results of the above project, albeit in close association with the supervisors. The student should be motivated to learn the different techniques, which are established protocols in the lab. We will work towards the student becoming more independent over the eight months in both the practical aspects of the project as well as in the ability to interpret the obtained data. 41 Lead Supervisor: Dr. Maarten Hoogenkamp Contact Email: Telephone: m.hoogenkamp@bham.ac.uk Co Supervisor: Dr. Vesna Stanulovic Project Title: The role of LMO proteins in neuroblastoma Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Neuroblastoma is one of the major types of cancers affecting young children and arises from cells of the sympathetic nervous system. Recently it was shown through a genome wide association study that LMO1 is involved in neuroblastoma. LMO1 is a protein that does not bind DNA directly, but is a component of particular DNA binding complexes, and its oncogenic potential has already been observed in the onset of T cell leukaemia. In neuroblastoma, higher expression levels of LMO1 were shown to lead to enhanced proliferation rates. Another LMO family member, i.e. LMO3, has already been implicated in neuroblastoma for a longer time. For both proteins their elevated expression corresponds to poor prognosis and more advanced disease. Within this project we want to check which LMO family members are expressed within the same cells, using a number of cell lines. We will perform pull down assays to see which proteins associate with LMO1, and set up a lentiviral system to knockdown LMO proteins within these cells. Combination knock-down of two LMOs may work better than only LMO1 to stop cells from growing or induce apoptosis. This would potentially be of therapeutic relevance because the LMOs are structurally very similar. This means a drug could be designed which interacts with a vital part of the LMO protein that is structurally the same between the members, thereby targeting more than one, functionally distinct, components of the cancerous phenotype. References Wang K. et al. (2011) Nature 469, 216-220 Ferronha et al. (2013) J. Neurosc. 33, 2773-2783 Aoyama et al. (2005) Cancer Res. 65, 4587-4597 How are you planning to ensure adequate supervision? 42 Both listed supervisors spend a significant proportion of their time at the bench in the laboratory, are skilled in the techniques mentioned above, and have prior experience in supervision of staff and students. This ensures that supervision and support is available on a daily basis throughout the eight months project. Every technique will initially be performed together with a supervisor, hopefully resulting in more independence later into the project. In addition there will be a weekly meeting to overview and discuss the progress and next steps, or problems that have been encountered. A weekly journal club will be held in which current literature on this and related topics will be discussed. The student role. The student is expected to be motivated and to actively participate in the research group. The student will be primarily responsible for performing the experiments and interpreting the results of the above project, albeit in close association with the supervisors. The student should be motivated to learn the different techniques, which are established protocols in the lab. We will work towards the student becoming more independent over the eight months in both the practical aspects of the project as well as in the ability to interpret the obtained data. 43 Lead Supervisor: Dr Marie-Christine Jones Contact Email: Telephone: m.c.jones@bham.ac.uk 48188 Co Supervisor: n/a Project Title: Overcoming platinum resistance in lung cancer by targeting cancer stem cells with combinations of chemotherapy with natural plant extracts. Pharmacy and Therapeutics Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Platinum drugs, alone or in combination, remain first line agents against non-small cell lung cancer. However, development of drug resistance may limit the efficacy of these agents over time. The exact mechanisms leading to platinum resistance are not fully understood but increasing evidence supports a role for cancer stem cells (CSCs). CSCs are thought to be mostly chemoresistant as they exploit different pathways to avoid the damage induced by anticancer drugs. Due to the ability of nanoparticles to bypass some of these resistance mechanisms, it can be expected that encapsulation of chemotherapeutic agents in nanoparticles could prevent the development of drug resistance and the proliferation of CSC. In parallel, natural plant extracts, including curcumin (the curry spice) have been shown to target CSCs. The objectives of this project are two-fold. Firstly, the aim is to determine the fraction of CSC in a common non-small lung cancer cell line and establish how that population changes following treatment with platinum drugs. Secondly, nanoparticles formulation of platinum-derivatives alone or combined with curcumin (turmeric extract; curry) or resveratrol (red wine) can prevent the enrichment of CSCs exposed to platinum drugs, overcome resistance and improve cytotoxicity. References [1] Barr MP, Gray SG, Hoffmann AC, Hilger RA, Thomale J, O’Flaherty JD, et al. Generation and Characterisation of Cisplatin-Resistant Non-Small Cell Lung Cancer Cell Lines Displaying a Stem-Like Signature. PLoS ONE. 2013;8:e54193. [2] Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? J. Pathol. 2011;223:147-61. [3] Gottschling S, Schnabel PA, Herth FJ, Herpel E. Are we missing the target? Cancer stem cells and drug resistance in non-small cell lung cancer. Cancer Genomics Proteomics. 2012;9:275-86. [4] Li Y, Zhang T. Targeting cancer stem cells by curcumin and clinical applications. Cancer Lett. 2014;346:197-205. [5] Sun TM, Wang YC, Wang F, Du JZ, Mao CQ, Sun CY, et al. Cancer stem cell 44 therapy using doxorubicin conjugated to gold nanoparticles via hydrazone bonds. Biomaterials. 2014;35:836-45. How are you planning to ensure adequate supervision? Active support will be provided at every step starting from experimental design to data analysis, as required, by 1) 2) 3) 4) 5) 6) 7) 8) 9) Providing adequate facilities for the work to be conducted Clearly defining the objectives of the project Identifying supervision needs Providing sufficient background literature to put the project in context Providing support in experimental design Providing technical support as required during the experiments Providing guidelines for data analysis and report writing Arranging regular meetings to follow progress Making sure any arising issues are addressed in a timely fashion The student role. The student is expected to conduct the cell culture experiments, analyse, interpret and discuss the data generated. The student will be trained in using the different techniques required for completion of the project. The student is also expected to provide updates on project progression and participate actively in planning and designing experiments. 45 Lead Supervisor: Nick Jones Contact Email: Telephone: n.d.jones@bham.ac.uk 43923 Co Supervisor: David Withers Project Title: Characterisation of innate lymphoid cell repopulation in a mouse model of bone-marrow transplantation MRC Centre for Immune Regulation Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Graft versus host disease (GVHD) is a common side-effect following allogeneic haematopoietic stem cell (HSC) transplantation and is a major cause of mortality and morbidity amongst the 30,000 recipients with haematological malignancies transplanted annually, worldwide. GVHD is mediated by donor T cells within the HSC transplant however the same cells maximise the graft versus tumour effect of the treatment. Therefore, medications to treat GVHD can increase susceptibility to tumour relapse (in addition to opportunistic infections). It has been recently shown that the presence of activated, donor-derived innate lymphoid cells (ILC) and the presence of ILC3 (a sub-type of ILC expressing RORin the peripheral blood correlated with decreased incidence of GVHD in patients receiving an allogeniec HSC transplant (Munneke, 2014). Furthermore, Hannash et al found that recipient ILC3 produce IL-22 which limits GVHD in a mouse model of GVHD (Hanash, 2012). Although both studies highlighted the potential of ILC3 to control GVHD it remains unclear as to the kinetics of ILC3 repopulation after transplantation and the relative importance of donor versus recipient ILC3. The proposed project will evaluate the reconstitution/proliferation and activation of the ILC compartment (with a focus on ILC3) following lethal irradiation and ILC3-GFP bone-marrow transplantion in mice. Using ILC3 knockout bone-marrow we will determine the impact of loss of donor ILC3 on alloreactive T cell responses and the induction of acute GVHD. The student will study a model of bone-marrow transplantation where residual ILC populations and recipient repopulation by donor ILCs can be followed after bone-marrow transplantation. To this end, T cell depleted (TCD) Ror -GFP (where ILC3 will express GFP) bone-marrow will be injected into lethally-irradiated C57BL/6xBALB/c F1 (CB6F1; H2d/b) recipients. Recipients will be analysed at multiple time-points following bone-marrow transplantation and lymphoid and non-lymphoid tissues analysed for the proportion/number/phenotype/activation status and localisation of ILCs by polychromatic flow cytometry and multi-colour confocal microscopy. Mice that have received bone-marrow transplantation will be compared to non-irradiated wild-type control mice. In order to assess the impact of donor-derived ILC3 populations on the T cell response to the recipient (that results in GVHD), irradiated F1 recipient mice will receive TCD bone-marrow from Ror -knockout mice (unable to generate ILC3) together with GVHD-inducing CFSE+CD45.1+ TEa T cells (TCR-transgenic CD4+ T cells that recognise the recipient MHC+peptide). The response of TEa T cells will be followed by flow cytometry and immunohistochemistry both in lymphoid tissues and GVHD target tissues (gut, skin and liver) and the kinetics of disease followed. 46 Overall, these studies will provide vital information regarding the re-population of ILCs in bone-marrow transplant recipients and will determine whether donor ILC3 seeding alters the subsequent T cell response to the recipient and induction of GVHD. References McKenzie, A.N., H. Spits, and G. Eberl. 2014. Innate lymphoid cells in inflammation and immunity. Immunity 41:366-374. Munneke, J.M., A.T. Bjorklund, J.M. Mjosberg, K. Garming-Legert, J.H. Bernink, B. Blom, C. Huisman, M.H. van Oers, H. Spits, K.J. Malmberg, and M.D. Hazenberg. 2014. Activated innate lymphoid cells are associated with a reduced susceptibility to graft-versus-host disease. Blood 124:812-821 Hanash, A.M., J.A. Dudakov, G. Hua, M.H. O'Connor, L.F. Young, N.V. Singer, M.L. West, R.R. Jenq, A.M. Holland, L.W. Kappel, A. Ghosh, J.J. Tsai, U.K. Rao, N.L. Yim, O.M. Smith, E. Velardi, E.B. Hawryluk, G.F. Murphy, C. Liu, L.A. Fouser, R. Kolesnick, B.R. Blazar, and M.R. van den Brink. 2012. Interleukin-22 protects intestinal stem cells from immune-mediated tissue damage and regulates sensitivity to graft versus host disease. Immunity 37:339-35 How are you planning to ensure adequate supervision? Initially I will meet with the student on a weekly basis with a joint Jones/Withers lab meeting once a month to discuss student progress and ideas pertaining to this project. In addition, the student will be directly supervised on a day to day basis by an experienced post-doctoral researcher (Kyoko Nakamura) as well as PhD students from both Jones and Withers laboratories. We will review this arrangement every two months and alter according to progress and required input from Dr Withers or myself. The student role. The student will be based within the MRC Centre for Immune Regulation in the IBR. Importantly, the IBR houses over 300 scientists working on an array of different immune processes, creating a hub of technological expertise and an interactive research environment. The student will have access to and training in key technologies within the College’s Core Technology Hub including advanced confocal microscopy, flow cytometry, cell sorting and a range of molecular techniques to support this project. The student will work with mouse tissue but will not perform in vivo experiments directly. In vivo experiments will be done in collaboration with Claire Dempsey who is undertaking a PhD employing the aforementioned mouse model of GVHD. The student will be expected to design, perform, analyse and interpret their own experiments with the help of experts in the Jones and Withers group. All necessary techniques have been established in either the Jones or Withers group and the student will be taught by researchers well versed in such techniques. Training on specialised equipment will be carried out either by laboratory members or by dedicated members of staff as appropriate. 47 The student will participate in a weekly departmental meeting in addition to supervisory 1 to 1 or lab meetings. The student will also be encouraged to attend postgraduate PDR activities such as seminars (such as John Squire, Happy Hour and CIIC) and journal clubs. It is also expected that the student will produce a first-rate project report where data is presented in either graph or photomicrograph form, the correct statistical evaluation of data has been used, the data has been robustly analysed without over-interpretation and that the data is discussed in the context of the wider literature. It is expected that the report is consistent with publication standards. 48 Lead Supervisor: Dr Nils P Krone Contact Email: Telephone: n.p.krone@bham.ac.uk 0121 414 2540 Co Supervisor: Mr Graham Fews GRAHAM.FEWS@bwnft.nhs.uk Project Title: Deciphering novel genetic variations associated with disorders of sex development Department: Centre for Endocrinology, Diabetes and Metabolism School for Clinical and Experimental Medicine Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Disorders of Sex Development (DSD) are a heterogenous group of conditions. Currently only approximately 40% of patients with DSD have a molecular genetic diagnosis following classic diagnostic pathways. Patients typically present either in the newborn period with atypical genitalia which may prevent immidiate gender assignment; or during adolescence where atypical sex development may become apparent. Current genetic testing strategies include chromosome analysis to establish genetic sex, specific gene mutation testing as directed by biochemical profiles and microarray analysis to detect genomic imbalances. The West Midlands Regional Genetics Laboratory has recently validated a next generation sequencing based gene panel to sequence 35 genes known to be causative of various DSD and identified novel mutations and clarified the diagnosis in atypical cases, thus providing personalised care. This includes gender assignment, stratified hormonal therapy and information on potential future risk for gonadal malignancies. This current diagnostic approach has already identified genetic variations in multiple genes in about 25% of patients with DSD. Whilst our strategy for genetic testing in DSD has already increased the effectiveness of genetic testing and provides an increased diagnosis, a significant proportion (about 30-40%) of patients will remain without a confirmed molecular basis for the underlying condition. Thus, the genetic service has developed novel tests to analyse genes involved in DSD by clinical exome sequencing to screen for mutations in genes known to be associated with sex development. It will be vital to correlate the genetic findings with the detailed clinical phenotype and model the functional consenquences of unknown gene variants to fully understand the clinical conquences. Thus this translational project has three main tasks: 1. To assists the deep phenotyping of patients with DSD to collect standardised clinical data. 2. To analyse molecular genetic data generated by next generation sequening. 3. To perform funtional in vitro analysis of novel genetic variants identified in patients with DSD Task 1: Patients are ongoing recruited from the multidiciplinary Disorder of Sex Development clinic (Clinical Lead Dr Krone) at Birmingham Children's Hospital (BCH). Phenotypic data will be collected into the clinical data base at BCH and core data will be also entered into the international I-DSD registry. The detailed examination will included the exact phenotypic charaterisation of the external genitalia, examination for additional dysmorphism and other clinical problem. In addition, data of examinations under anaesthesia (EUA), ultrasounds, MRI and histological data will be 49 collected. Blood sampling for molecular genetic analysis is part of the standard care pathway for these patients. DNA samples will be collected via phlebothomy and send to the West Midlands Regional Genetics Laboratory for genetic analysis using NGS approaches. Task 2: Patient DNA will be stored and prepared for running on the Illumina TruSight One Sequencing Panel and run on an Illumina HiSeq NGS system in the the West Midlands Regional Genetics Laboratory (Mr G Fews). The resulting sequencing data will be processed and analysed for variation against the genomic reference sequence to ascertain mutations considered to be pathogenic and clinically significant to the DSD. These mutations will then be confirmed by Sanger sequencing. Task 3: The genetic findings will be discussed at multidiplinary meetings and pathogenic mutations will be analysed in vitro by functional expression analysis, to characterise multiple hits within pathways involved in sex development. Mutations will be introduced in respective cDNAs by in vitro mutagenesis. These will be transfected into mammalian cell culture models to assess the in vitro protein properties of respective. If transcription factors are involved, we will assess in vitro transactivation potential towards respective receptors; in case of steroidogenic enzymes the capacity to converst steroid hormones will be assessed. These studies can be complemented by recreating mutations in vivo and study their effects in zebrafish models. This project is vital for the development of novel clinical pathways in rare genetic disease as it will promote our understanding regading the clinical consequences of genetic variants associated with disorders of sex development. References Kolesinska Z, Ahmed SF, Niedziela M, Bryce J, Molinska-Glura M, Rodie M, Jiang J, Sinnott RO, Hughes IA, Darendeliler F, Hiort O, van der Zwan Y, Cools M, Guran T, Holterhus PM, Bertelloni S, Lisa L, Arlt W, Krone N, Ellaithi M, Balsamo A, Mazen I, Nordenstrom A, Lachlan K, Alkhawari M, Chatelain P, Weintrob N. Changes over time in sex assignment for disorders of sex development. Pediatrics. 2014 Sep;134(3):e710-5. Bashamboo A, McElreavey K. Gene mutations associated with anomalies of human gonad formation. Sex Dev. 2013;7(1-3):126-46 Ahmed SF, Achermann JC, Arlt W, Balen AH, Conway G, Edwards ZL, Elford S, Hughes IA, Izatt L, Krone N, Miles HL, O'Toole S, Perry L, Sanders C, Simmonds M, Wallace AM, Watt A, Willis D. UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development. Clin Endocrinol (Oxf). 2011 Jul;75(1):12-26 How are you planning to ensure adequate supervision? The proposed project is well embedded into ongoing research to model and understand novel genetic variations in genes associated with disorders of sex development. These projects are funded by European charities (IFCAH-ESPE) and the EU fp7 framework program. Overarching structured supervision will formally take place at least once weekly during lab meetings and on an informal basis when required with the supervisors. To guarantee the maximum scientific outcome (presentations, publications) and the best possible work experience (acquiring scientific skills and broad cutting edge methods) the student will be able to get all required support during daily interaction with postdocs working on related projects in the lab. The student role. 50 Throughout the project, the student will grow into the role with support of our research teams to perform experiments with an increasing level of independence. Together with the student, we will develop experimental outlines to enforce a successful outcome of the proposed studies. The project has three key elements embarking on a translational research philosophy. The student will be able to attend multidisciplinary team clinics for Disorders of sex development at Birmingham Children’s Hospital (Clinical Lead, Dr Krone) and the student will be involved in the systematic collection of phenotypic data. In addition, the student will be able to take part in the data analysis by next generation sequencing in the Clinical Genetics Units (Principal Geneticist, Mr Fews) and learn cutting edge methods of genetic analysis. Mutations of unknown relevance will be modelled in vitro in Dr Krone’s lab at the Centre of Endocrinology, Diabetes and Metabolism (CEDAM) across the road from the genetics units. Over the course of the project, the student will acquire a multitude of generally applicable lab methods including PCR, cloning, plasmid DNA (MiniPrep, MidiPrep) and RNA preparation, transformation, transfection, cell culture techniques fluorescence microscopy, mRNA synthesis and purification. These methods will equip the student with the required skill mix to gain independence during the daily work. This will also provide the vital basis for a potential career in academic medicine. In addition, the student will acquire basic skill in cutting edge technologies such a steroid metabolome analysis by LC-MS/MS. We see the student as an integral member of our research teams and will provide them with state-of-the-art experience and knowledge to master projects and in translational medicine. 51 Lead Supervisor: Dr Nils P Krone Contact Email: Telephone: n.p.krone@bham.ac.uk 0121 414 2540 Co Supervisor: Dr Grareth G Lavery g.g.labery@bham.ac.uk Project Title: The role of adrenodoxin in redox regulation of steroid hormone biosynthesis Department: Centre for Endocrinology, Diabetes and Metabolism School for Clinical and Experimental Medicine Will the project require a Home Office working with animals licence? Not necessary, but preferred Is the Project Cancer related? No Project Outline Background: Steroid hormones are key regulators of sex development, homeostasis, and metabolism. Of the Cytochrome P450 (CYP) enzymes, CYP type-1 enzymes are active in the mitochondrion relying on electron transfer from adrenodoxin (ADX) and adrenodoxin reductase (ADR). CYP type-2 enzymes are localised to the endoplasmic reticulum and depend on P450 oxidoreductase as electron donor. ADX/ADR dependent reactions account for more than half the synthesis steps in steroid hormone production. Variations in mitochondrial redox regulation of steroidogenesis are hypothesised to account for phenotypic differences in common disease such as hypertension and modulators of phenotypic expression in inborn errors of steroidogenesis. However, redox regulation of mitochondrial steroidogenesis has not been studied in vivo, and in contrast to microsomal steroidogenesis, no human mutations have been described in the mitochondrial cofactors ADX and ADR. This project will elucidate the critical mechanisms regulating in vivo mitochondrial steroidogenesis vitally important for understanding of range health and disease. Activation of such pathways are likely to increase reactive oxygen species and apear to be an attractive target in the treatment of hormone depedent cancers. Hypothesis: Adrenodoxin control of CYP type-1 enzymes in the mitochondria is central to the control of in vivo steroidogenesis Experimental Design and Methods: We have recently created a global Adxdeletion and heterozygous Adx-deletion allele mice are readily available and will be used to breed a global Adx knock-out mouse. We will breed litters to study if the mutation has an impact on pregnancy or development according to Mendelian inheritance. An impairment of pregnancy is unlikely as progesterone, required to maintain pregnancy, is produced by the corpus luteumand not by the placenta like in humans from mid-pregnancy. Adrenodoxin expression will be assessed by Western Blot verifying adrenodoxin deficiency. As a next step, thorough macroscopic analysis of size and growth of pups, organ size and weight with focus on the adrenal and gonad will be conducted. The phenotype of adrenocortical insufficiency might vary similar to effects observed in the deletion model of sterodoidogenic acute regulatory protein (star) deletion mouse. Histological methods will use H&E and oil red staining as well as immunohistochemistry for adrenodoxin, adrenodoxin reductase, cytochrome P450 enzymes and transcription factors involved in regulation of expression of steroidogenic enzymes. We will assess the functional consequences of altered electron provision towards mitochondrial steroidogenesis by analysing plasma and urine by LC/MSMS and GC/MS, which are a well established methods in our laboratory and their key regulatory hormones 52 (ACTH, gonadotrophins). Finally, we will conduct ex vivo whole organ cultures of the adrenals and gonads. Employing such an approach will allow us to dissect the differential effects of an adrenodoxin deletion on steroidogenic pathways by incubation with defined steroid precursors specific for different CYP type 1 conversion reactions. These studies will allow insight into the differential impairment of CYP type 1 enzymes (cyp11a1, cyp11b1, cyp11b2) in an intact organ system. Outcome: This project will generate unique data on the physiological role of adrenodoxin. The work is well embedded into current research of both supervisors will set the basis for further work to generate adrenal and gonadal specific Adxdeletion models. It will provide a postgraduate student with a huge skill set in animal research and research publications. References Miller WL, Auchus RJ 2011 The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81-151 Krone N, Arlt W 2009 Genetics of congenital adrenal hyperplasia. Best practice & research 23:181-192 Parajes S, Kamrath C, Rose IT, Taylor AE, Mooij CF, Dhir V, Grötzinger J, Arlt W, Krone N 2011 A novel entity of clinically isolated adrenal insufficiency caused by a partially inactivating mutation of the gene encoding for P450 side chain cleavage enzyme (CYP11A1). J Clin Endocrinol Metab 96:E1798-1806 Lisurek M, Bernhardt R 2004 Modulation of aldosterone and cortisol synthesis on the molecular level. Mol Cell Endocrinol 215:149-159 How are you planning to ensure adequate supervision? The proposed project is well embedded into ongoing research to model and understand steroidogenesis and steroid hormone metabolism. These projects are funded by European charities (IFCAH-ESPE), the EU fp7 framework program and the Wellcome Trust. Overarching structured supervision will formally take place at least once weekly during lab meetings and on an informal basis when required with the supervisors. To guarantee the maximum scientific outcome (presentations, publications) and the best possible work experience (acquiring scientific skills and broad cutting edge methods) the student will be able to get all required support during daily interaction with postdocs working on related projects in the lab. The student role. Throughout the project, the student will grow into the role with support of our research teams to perform experiments with an increasing level of independence. Together with the student, we will develop experimental outlines to enforce a successful outcome of the proposed studies. Over the course of the project, the student will acquire a multitude of generally applicable lab methods including PCR, cloning, plasmid DNA (MiniPrep, MidiPrep) and RNA preparation, transformation, transfection, cell culture techniques bright fied and fluorescence microscopy, mRNA synthesis and purification. These methods will equip the student with the required skill mix to gain independence during the daily work. This will also provide the vital basis for a potential career in academic medicine. In addition, the student will acquire basic skill in cutting edge technologies such a steroid metabolome analysis by LC-MS/MS. We see the student as an integral member of our research teams and will provide them with state-of-the-art experience and knowledge to master projects and in translational medicine. 53 Lead Supervisor: Dr Patricia F. Lalor Contact Email: Telephone: p.f.lalor@bham.ac.uk x46967 Co Supervisor: Professor Stefan Hubscher Project Title: Prognostic and functional significance of hepatic expression of L-FABP and varL-FABP Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? - Yes Project Outline Liver fatty acid binding protein (L-FABP or FABP-1) is expressed in the pancreas, small intestine, kidney and the liver. It is involved in intracellular transport and chaperone functions for long chain fatty acids, and maintains appropriate cytosolic concentrations of fatty acid. Cytoplasmic expression is reportedly decreased in individuals with moderate to severe NASH [1] compared to those with simple steatosis, whilst other studies suggest an increase in expression in individuals with diabetes and metabolic syndrome. A recent study has shown that polymorphisms of FABP1 make an individual more susceptible to NAFLD [2] and also to developing type II diabetes[3], and we have shown that it is possible to detect both variant and native protein in the livers of individuals with NASH using novel MS technology[4]. Recent evidence also suggests that l-FABP is a valuable tool for staging hepatic adenomas[5] and colorectal neoplasms[6] but to date little data exists for expression of variant forms during malignancy. Therefore this project will use histochemical and molecular techniques to investigate the expression of native and variant L-FABP in hepatic disease. References 1. 2. 3. 4. 5. 6. Charlton, M., et al., Hepatology., 2009. 49(4): p. 1375-84. Peng, X.E., et al., Gene., 2012. 500(1): p. 54-8. Epub 2012 Mar 20. Mansego, M.L., et al., PLoS One., 2012. 7(3): p. e31853. Epub 2012 Mar 2. Sarsby, J., et al., J Am Soc Mass Spectrom, 2014. 25(11): p. 1953-61. van Aalten, S.M., et al., J Hepatol, 2011. 55(1): p. 120-5. Lawrie, L.C., et al., Br J Cancer, 2004. 90(10): p. 1955-60. How are you planning to ensure adequate supervision? We have chosen to combine the technical expertise of experienced clinical and academic supervisors to ensure the student is exposed to the maximal number of transferable research skills and has access to appropriate clinical samples. All supervisors have a proven track record in supervision of both undergraduate and postgraduate students, and prior experience supervising successful Intercalation projects. For example one of Dr Lalor’s students has been awarded a FALK/Core award for his research project (2014 – Ding Yang) Appropriate training in research techniques and data analysis and presentation will be supplied. We will have weekly meetings with supervisors and interactions with other members of the research groups, who will be available day to day for advice and tuition will be encouraged. The student role. 54 The student will be expected to perform experiments and conduct data analysis. They will present data to the supervisory team and wider research groups and be expected to assimilate available published literature under guidance from supervisors. They will be trained and expected to work using good laboratory practice under local standard operating procedures and be mindful of ethical use of human tissue specimens. They will be working in large research teams and thus would be expected to interact with colleagues and use local facilities in a respectful manner. 55 Lead Supervisor: Dr Patricia F. Lalor Contact Email: Telephone: p.f.lalor@bham.ac.uk x46967 Co Supervisor: Dr Tariq Iqbal Project Title: The consequences of mucosal platelet activation in IBD Department: Liver research Laboratory, I+I Will the project require a Home Office working with animals licence? No Is the Project Cancer related? - no Project Outline Platelets are traditionally considered as having a primary role in coagulation, however studies from our group and others confirm that platelets can, under some circumstances bind using integrins to endothelial cells where they can support leukocyte recruitment to the vessel wall[1, 2]. They have beneficial roles in haemostasis [3], pathogen clearance during sepsis[4], production of serotonin to assist wound healing in multiple organs. Inflammatory bowel disease is associated with increased platelet activation and risk of thromboembolism [5], and patients commonly have elevated counts of small platelets [6, 7]with an activated phenotype[8, 9] and high levels of CD40L expression. This is thought to contribute to elevated levels of circulating CD40L found in patients and aggregation of platelets in gastric mucosal tissue[7]. The importance of platelets to disease pathogenesis is illustrated by studies showing that platelet-derived miRNA biomarkers are identified in screens of inflammatory bowel disease susceptibility genes[10]. Importantly use of Clopidogrel to block platelet function has shown promise for reduction of disease burden in animal models of colitis and Crohns disease[11] and retrospective analysis of patients suggests reduced disease activity in patients treated with anti-platelet agents[12]. Currently existing drugs which target the recruitment and activation of platelets in the bowel would have promise in the treatment of IBD. However there is a risk of bleeds and possible disturbance of normal mucosal anatomy in patients taking aspirin and other antiplatelet agents[13] and thus whist of definite potential[14], this explains the reluctance to adopt this therapeutic strategy. Importantly the mechanisms of platelet activation in diseased bowel are not well characterized and nor are the mechanisms by which the platelets bind to the colonic vasculature. Additionally no studies in humans have demonstrated their contribution to bowel inflammation. Identification of key anti-adhesive pathways would allow targeting of anti-platelet therapies to the bowel, thereby minimizing compromise of coagulation. We have demonstrated the efficiacy of this strategy I previos studies of the hepatic microenvironment[2] In this study we will use functional assays and immunocytochemical techniques to report the regulation of platelet activation and adhesion within the bowel. References 1. 2. 3. Lalor, P. and G.B. Nash, Br.J.Haematol., 1995. 89(4): p. 725-732. Lalor, P.F., et al., American journal of physiology. Gastrointestinal and liver physiology, 2013. 304(5): p. G469-78. Pereboom, I.T., T. Lisman, and R.J. Porte, Liver transplantation : 2008. 14(7): p. 923-31. 56 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. Ma, A.C. and P. Kubes, Journal of thrombosis and haemostasis : JTH, 2008. 6(3): p. 415-20. Webberley, M.J., M.T. Hart, and V. Melikian, Gut, 1993. 34(2): p. 247-51. Larsen, T.B., et al., Pathophysiology of haemostasis and thrombosis, 2002. 32(2): p. 92-6. Collins, C.E., et al., European Journal of Gastroenterology & Hepatology, 1997. 9(12): p. 1213-7. Collins, C.E., et al., Gastroenterology, 1994. 106(4): p. 840-5. Danese, S., et al., Gut, 2003. 52(10): p. 1435-41. Duttagupta, R., et al., PloS one, 2012. 7(2): p. e31241. Patel, S.H., M.A. Rachchh, and P.D. Jadav, Indian journal of pharmacology, 2012. 44(6): p. 744-8. Vinod, J., et al., Journal of clinical gastroenterology, 2012. 46(6): p. 527-9. Taha, A.S., et al., Alimentary pharmacology & therapeutics, 2006. 23(4): p. 489-95. Pitchford, S.C., British journal of pharmacology, 2007. 152(7): p. 987-1002. How are you planning to ensure adequate supervision? We have chosen to combine the technical expertise of experienced clinical and academic supervisors to ensure the student is exposed to the maximal number of transferable research skills and has access to appropriate clinical samples. All supervisors have a proven track record in supervision of both undergraduate and postgraduate students, and prior experience supervising successful Intercalation projects. For example one of Dr Lalor’s students has been awarded a FALK/Core award for his research project (2014 – Ding Yang) Appropriate training in research techniques and data analysis and presentation will be supplied. We will have weekly meetings with supervisors and interactions with other members of the research groups, who will be available day to day for advice and tuition will be encouraged. The student role. The student will be expected to perform experiments, collect tissue samples from clinic and conduct data analysis. They will present data to the supervisory team and wider research groups and be expected to assimilate available published literature under guidance from supervisors. They will be trained and expected to work using good laboratory practice under local standard operating procedures and be mindful of ethical use of human tissue specimens. 57 Lead Supervisor: Dr Steven Lee Contact Email: Telephone: s.p.lee@bham.ac.uk (tel 0121-414-2803) Co Supervisor: Prof Roy Bicknell Project Title: Targeting the tumour vasculature with genetically engineered T cells. Department: School of Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Compared to normal tissue, angiogenesis in tumours is deregulated and/or aberrant, resulting in a structurally and functionally abnormal vasculature. Targeting unique features of the tumour vasculature to compromise blood flow in tumour tissue should therefore provide therapeutic benefit. Anti-angiogenic monoclonal antibodies or small molecules that target these tumour endothelial markers appears to have limited curative potential, possibly because of their cytostatic action and the redundancy of angiogenic pathways. In principle, cytotoxic strategies should be more effective because they could prevent formation of new vessels and destroy existing tumour vasculature. T lymphocytes are self-replicating effectors that can persist for years and display potent and specific cytotoxic activity. Recent clinical studies infusing cytotoxic T lymphocytes (CTLs) specific for antigens expressed on malignant cells have demonstrated remarkable efficacy in treating metastatic melanoma1. However, extending this therapy to other cancers is limited by a lack of appropriate tumour antigens. Targeting T cells to tumour endothelial markers offers an alternative approach that in animal models has been shown to inhibit tumour growth and prolong host survival2,3. Melanoma Cell Adhesion Molecule (MCAM) was originally identified as an antigen on metastatic melanoma cells4 and is thought to play a role in cell adhesion. More recently, following an extensive analysis of renal cell carcinoma tissues, we have found that MCAM is also a tumour endothelial marker, highly expressed on the vasculature of renal cell carcinoma but absent or poorly expressed in normal tissues (Fig. 1) (Wragg & Bicknell, unpublished data). Fig. 1 MCAM is highly expressed on blood vessels within renal cell carcinoma tissue (indicated by brown staining) but not in adjacent normal tissue. Normal tissue Tumour tissue 58 To date naturally occurring T cell responses to MCAM have not been described, but it is possible to engineer T cells with a defined specificity by transducing genes encoding “chimeric antigen receptors” (CARs). CARs combine the specificity of antibodies with the cytotoxic and immunomodulatory functions of T cells and operate in an MHC-unrestricted manner (reviewed5). Typically, CARs consist of a single chain variable fragment (scFv) from a specific antibody linked to intracellular T cell signalling domains (Fig. 2). The anti-tumour effects of CAR-expressing T cells have been demonstrated in preclinical models6 and more recently with some dramatic results in clinical trials7-9. Using the ETH-2 human antibody phage library10 we plan to isolate antibodies specific for human MCAM, and then to generate CAR constructs that will target T cells to recognise and destroy the tumour vasculature. Aims: Generation and characterisation of MCAM-specific CARs to assess their therapeutic potential for cancer. The project will focus on the following areas: 1. Using our phage antibody library, we will use recombinant human MCAM to isolate antibodies specific for this molecule. 2. Using molecular techniques, single chain variable fragment (scFv) genes that encode these MCAM-specific antibodies will be cloned into an existing retroviral expression plasmid designed to generate the CAR construct. 3. Human T cells will be transduced in vitro using these retroviruses to stably express the CAR on the cell surface. Expression of the CAR will be explored using flow cytometry. 4. To explore the specificity and function of these CAR-expressing T cells they will be tested for their ability to proliferate and release cytokines in response 59 to purified MCAM and their ability to kill MCAM-expressing target cells. 5. If in vitro data look promising and if time permits, preliminary studies will be conducted in vivo, using mouse tumour models to explore the safety and efficacy of this approach. This project is based on a joint study between two labs in the medical school, combining the expertise of Dr Steve Lee (T cell therapy including engineering T cells to express CARs) and Prof Roy Bicknell (Angiogenesis). References 1. Rosenberg, SA et al. Clin Cancer Res 17, 4550-4557, (2011). 2. Chinnasamy, D et al. J Clin Invest 120, 3953-3968, (2010). 3. Niederman, TM et al. Proc Natl Acad Sci U S A 99, 7009-7014, (2002). 4. Lehmann, JM et al. Cancer Res 47, 841-845, (1987). 5. Sadelain, M et al. Cancer Discov 3, 388-398, (2013). 6. Brentjens, RJ et al. Nat Med 9, 279-286, (2003). 7. Maude, SL et al. N Engl J Med 371, 1507-1517, (2014). 8. Kalos, M et al. Sci Transl Med 3, 95ra73, (2011). 9. Brentjens, RJ et al. Sci Transl Med 5, 177ra138, (2013). 10. http://www.pharma.ethz.ch/institute_groups/biomacromolecules/protocols/eth How are you planning to ensure adequate supervision? The student will be fully supported through scheduled weekly meetings with the primary supervisor to discuss experiments and any concerns the student may have. The student will work closely with the primary supervisor and have regular (almost daily) access to both him and members of the Lee and Bicknell labs. Day to day the student will work alongside other members of the Lee lab who have extensive experience of this type of work. The student role. During the project, the student will spend most of their time engaged in laboratorybased research. Under the supervision of Dr Lee and following a period of training, they will design, conduct and interpret the results of experiments. They will also have time to read around the subject area, to understand the background to the project and to keep up to date with recent developments. The student will take an active role in weekly lab meetings/journal clubs in which they will have a chance to discuss their own data and critique the work of others. 60 Lead Supervisor: Dr Felicity de Cogan Contact Email: Telephone: 0121 4144859 Co Supervisor: Prof Ann Logan Project Title: Self-cleaning surfaces: Preventing the spread of hospital infection Department: School of Clinical and Experimental Medicine Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Nosocomial (i.e. hospital acquired) infections affect 1 in 11 hospital patients at any one time and cost the NHS approximately £1 billion year1. The most common way of spreading infection is through contaminated surfaces such as beds, taps and door handles. While hospital infection controls such as hand washing, personal protective equipment and patient isolation can reduce the risk of infection, these approaches are inadequate. Our work has developed a simple, cheap and durable protein coating for different surfaces including metals, which prevents bacterial colonisation, using titanium (material most commonly used in orthopaedic implants) as a base material. The work carried out in this project will move this work away from orthopaedic implants and towards general hospital surfaces, such as door handles, medical equipment and surgical devices. The student will carry out synthetic biology techniques for protein synthesis. They will carry out surface attachment of the active proteins onto the target surfaces and then use microbiology assays to test the efficacy of the coating and identify and specify targeted bacteria and carrying out a randomised control trial of the surfaces in the Medical School infrastructure to test the anti-microbial efficacy of the surfaces in a ‘real’ environment. References 1. UK National Audit Office ‘The management and control of hospital acquired infection in acute NHS trusts in England, 17, February, 2000. How are you planning to ensure adequate supervision? The student will be trained and supervised on a daily basis by Dr Felicity de Cogan. The student will attend weekly group meetings to report back on the project to the entire group and obtain feedback and ideas. The student will also have regular progress/supervisory meetings with Professor Logan (Head of Section) and Dr Mark Webber (Bioscience Collaborator) about their work. The student role. 61 The student will carry out the day to day experimental work on the project. The student will gain familiarisation with terminology and literature and existing healthcare challenges in the field of antimicrobials and antibacterial resistance. They will learn and exploit novel methods of antimicrobial surface attachment. They will learn basic microbiology techniques and learn how to isolate and process pathogen samples and to identify and track bacterial species. The student will gain experience in carrying out a randomised controlled study and in blinded analysis. 62 Lead Supervisor: Christian Ludwig Contact Email: Telephone: c.ludwig@bham.ac.uk Co Supervisor: Jay Nath, Surgical Research Fellow & PhD Student. Project Title: Unlocking the metabolism of the kidney prior to transplantation. Department: School of Cancer Sciences with crossover with School of Immunity & Infection. Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Our research vision is to optimise the metabolic support during the machine perfusion of kidneys for transplantation to safely preserve an organ for 72 hours prior to transplantation. The development of hypothermic machine perfusion (HMP) has already demonstrated clinical benefit and is associated with reduced rates of Delayed Graft Function and improved graft survival in machine perfused kidneys compared to those preserved in traditional Static Cold Storage (1-2). There is increasing evidence that substantial metabolic activity occurs during HMP and may have a protective effect. However the metabolic activity in the ex-vivo, hypoxic, hypothermic environment provided by HMP is poorly understood. Initial work from our group, using 1D 1H-NMR spectroscopy has identified a panel of 28 key metabolites within the kidney perfusate. We have found that the metabolomic profile from perfusates of Immediate Graft Function kidneys differs from that of Delayed Graft Function. These changes are apparent as little as 45 minutes after perfusion commences (3,4). A greater understanding of the active metabolic pathways within the HMP kidney may allow a target for metabolic manipulation during perfusion. There is some evidence that broad metabolic support with cell culture like perfusate improves the viability of damaged porcine kidneys (5). We intend to perform further studies utilising 2D NMR techniques incorporating labelled metabolites into perfusion fluid to determine their metabolic fate. 13 C The student will join an active research team, investigating an exciting topic within renal transplantation. This study will facilitate a more complete understanding of the metabolic processes within a machine perfused kidney using novel 13C technology with the intention of developing a new metabolically supportive perfsion fluid. There is no similar published work in this field and would be an exciting opportunity for an aspiring academic clinician. This would be particularly suited to a canditate with an interest in surgery or transplantation. References 63 1. Moers C, Pirenne J, Paul A, Ploeg RJ, Machine Preservation Trial Study G (2012) Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med 366: 770-771. 2. Lodhi SA, Lamb KE, Uddin I, Meier-Kriesche HU (2012) Pulsatile pump decreases risk of delayed graft function in kidneys donated after cardiac death. Am J Transplant 12: 2774-2780. 3. Guy AJ, Nath J, Cobbold M, Ludwig C, Tennant DA, et al. (2014) Metabolomic Analysis of Perfusate During Hypothermic Machine Perfusion of Human Cadaveric Kidneys. Transplantation Sep 12. PMID: 25222017 4. Nath J, Guy A, Smith TB, Hodson J, Cobbold M, et al. (2014) Metabolomic perfusate analysis during kidney machine perfusion: the pig provides an appropriate model for human studies. PLOS One (publication date due 12 Dec). 5. Brasile L, Stubenitsky BM, Haisch CE, Kon M, Kootstra G (2005) Repair of damaged organs in vitro. Am J Transplant 5: 300-306. How are you planning to ensure adequate supervision? The student will work under the close supervision of the current final year PhD student who will oversee day-to-day training and will provide surgical training and full supervision during any animal kidney perfusion experiments. As well as the daily interactions with the research team, the student will be expected to attend regular departmental ‘lab meetings’, ensuring exposure to a range of disciplines. We will provide in house training on the fundamental concepts of NMR analysis and scientific manuscript preparation. The student role. The student will be given appropriate training and be expected to assist during pig kidney perfusion experiments, including visits to the abattoir with on-site perfusion. They will be given the surgical training for this and all experiment will be overseen by our surgical research fellow. They will be given training to and expected to perform basic laboratory tasks including perfusion fluid preparation, Cell extraction, sample preparation and Cell culture. A significant element of the project work relies on NMR analysis and the student will be trained and expected to process and interpret NMR spectra – (both 1D and various types of 2D NMR experiments). Our research group has been expanding our publication output and has produced 2 high impact papers over the past 6 months (ref 3 & 4). The student would be expected to contribute towards further manuscript preparation and we would expect them to attain a publication as a result of their BMedSc. 64 Lead Supervisor: Dr Yuk Ting Ma (Senior Clinical Lecturer) Contact Email: Telephone: y.t.ma@bham.ac.uk Co Supervisor: Dr Sarah Leonard (Post-Doctoral Fellow) Project Title: The use of a genome-wide haploid genetic screen to identify the critical genes that govern response to sorafenib in the treatment of hepatocellular carcinoma School of Cancer Sciences, University of Birmingham Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Loss-of-function genetic screens in model organisms have helped to elucidate many biological processes, but such large scale gene disruption has not been possible in human cells due to their diploid genome. Recently, a genome-wide loss-of-function screening method has been developed in a human haploid cell line (Carette 2009). In this approach, insertional mutagenesis was used to generate null alleles in a cancer cell line haploid for all chromosomes except chromosome 8 (KBM7). Haploid screening has recently been used to identify inter alia the critical mediator of tunicamycin toxicity, the host receptor for Clostridium difficile toxin, and the host factors important for Chlamydia trachmomatis infection (Reiling 2011, Papatheodorou 2011, Rosmarin 2012). This approach avoids the potential off-target effects and incomplete knockdowns of a siRNA screen, and has the advantage of causing only a limited number of disrupted genes per cell, which can be readily identified by deep sequencing. This haploid loss-of-function genetic screening method thus provides an exciting and novel strategy for identifying the critical genes that govern response to novel drugs or drug combinations. Hepatocellular carcinoma (HCC) is a leading cause of cancer death globally and its incidence is increasing in the West, with the increasing burden of chronic liver disease. Until recently, systemic treatment options for advanced disease were limited. However, randomised clinical trials have demonstrated that the multikinase inhibitor, sorafenib, prolongs survival in appropriately selected patients (Llovet 2008) and this drug has become the standard for patients with advanced HCC. However the effects of sorafenib are modest (2.8 months improvement in median survival), is associated with significant toxicity and is expensive (approx. £3000 per month per patient). Restricting the use of sorafenib to patients who will respond will improve its clinical effectiveness and thus cost-effectiveness, and will also spare unnecessary toxicity in patients who will not respond. However, there is currently no predictive biomarker of response. We have exposed gene-trapped mutagenized KBM7 cells to sorafenib and surviving (resistant) clones have been expanded, harvested and genomic DNA extracted. The mutated genes are currently being identified by mapping of the insertion sites using high throughput sequencing. References 65 Carette JE, Guimaraes CP, Varadarajan M, et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science 2009; 326: 1231-1235. Llovet JM, Ricci S, Mazzaferro V et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008; 359: 378-390. Papatheodorou P, Carette JE, Bell GW, et al. Lipolysis-stimulated lipoprotein receptor (LSR) is the host receptor for the binary toxin Clostridium difficile transferase (CDT). Proc Natl Acad Sci U S A 2011; 108: 16422-16427. Reiling JH, Clish CB, Carette JE, et al. A haploid genetic screen identifies the major facilitator domain containing 2A (MFSD2A) transporter as a key mediator in the response to tunicamycin. Proc Natl Acad Sci U S A 2011; 108: 11756-11765. Rosmarin DM, Carette JE, Olive AJ, et al. Attachment of Chlamydia trachomatis L2 to host cells requires sulfation. Proc Natl Acad Sci U S A 2012; 109: 10059-10064. How are you planning to ensure adequate supervision? The lead supervisor, Dr Ma, will meet with the student weekly. Dr Leonard will supervise the student in the laboratory on a daily basis. The student role. In this project, the student will perform the validation experiments on selected candidate genes identified from the haploid genetic screen described above. To confirm that the gene trap insertion of the affected locus is responsible for the observed resistance to sorafenib in mutagenized KBM7 cells, individual resistant clones carrying the defined gene-trap insertion will be isolated. Absence of gene expression will be confirmed using immunoblotting, and the wild-type cDNA sequence of the gene of interest will then be ectopically expressed in these null-cells to assess if drug sensitivity can be restored. Candidate genes will then be examined for their conserved function in hepatocellular carcinoma cells by first determining if expression level correlates with cell line sorafenib sensitivity, and second, by knocking down or over-expressing the gene in sensitive and resistant HCC cell lines, respectively. 66 Lead Supervisor: Konstantinos Manolopoulos Contact Email: Telephone: k.manolopoulos@bham.ac.uk 4147525 Co Supervisor: Gareth Lavery Project Title: Understanding adipose tissue dysfunction in obesity and type 2 diabetes Department: CEM-CEDAM Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Obesity is the leading cause of insulin resistance and type 2 diabetes. Adipose tissue dysfunction has been proposed as being central in the pathophysiology of obesityrelated complications. However, the exact mechanisms remain unclear. Furthermore, there is a big variation in the response to weight-loss promoting therapies in patients with type 2 diabetes, suggesting intrinsic differences in adipose tissue function between individuals. The aims of this project are two-fold: Firstly, to analyse a clinical database of obese diabetes patients receiving treatments known to promote weight loss (GLP-1 agonists, DPP-4 antagonists, SGLT-2 antagonists), and establish predictors of weight loss responses. Secondly, to characterise adipose tissue function in various degrees of obesity in a lab-based project. For this, adipose tissue biopsies obtained from a range of patients with obesity will be assessed in terms of gene and protein expression profiles, histomorphology and in vitro metabolic function. Lab methods will involve cell culture, RNA extraction and qPCR techniques, Western blots and in vitro assays of glucose and lipid metabolism. By linking the in vitro findings with the clinical data this project will help deepending our understanding of adipose tissue dysfunction in obesity and type 2 diabetes. References Shulman GI , Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014 Sep 18;371(12):1131-41. KN Manolopoulos, F Karpe, Frayn KN, Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond). 2010 Jun;34(6):949-59 How are you planning to ensure adequate supervision? Initial period of focused training in data analysis methodology and in vitro methods. Open door policy for day-to-day queries and support with data analysis and lab work. Weekly meetings to assess progress and provide support. The student role. Following an initial training period you would be expected to carry out the database analysis and apply statistical regression models to identify predictors of weight loss. In the lab, you would be responsible for your own cell cultures, and in vitro experiments in adipocytes derived from the patient biopsies. While you are expected to work independently, ample training and support will be provided. 67 Lead Supervisor: Dr Helen McGettrick Contact Email: Telephone: h.m.mcgettrick@bham.ac.uk 0121 414 4043 Co Supervisor: Dr Andrew Filer Project Title: Synovial fibroblasts release microvesicles with different bioactivity in acute and chronic inflammation: Role in regulating the inflammatory infiltrate? Rheumatology Research Group Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Rheumatoid arthritis (RA) is a prototype inflammatory disease in which synovial fibroblasts induce joint damage and maintain the persistence of the inflammatory episode [1]. Multiple epigenetic changes drive the acquisition of a pathogenic phenotype in rheumatoid synovial fibroblasts that underpins these aberrant behaviours. A window of opportunity exists during the first 3 months of RA when the disease is still evolving and more responsive to current treatment strategies [2,3]. This raises intriguing questions about the development of the fibroblast phenotype during the earliest stages of persistent arthritis. One way fibroblasts moderate the inflammatory infiltrate is by “talking” to neighbouring vascular endothelium. We have previously shown that rheumatoid synovial fibroblasts activate endothelium to inappropriately recruit leukocytes in vitro [4]. Using the Birmingham Early Arthritis Cohort (BEACON) we have recently shown that fibroblasts from acutely resolving arthritis are immunosuppressive, inhibiting lymphocyte adhesion to cytokine-treated endothelium in an IL-6 and TGFb dependent manner. Interestingly, this effect was lost in very early RA, such that fibroblasts no longer suppressed recruitment but rather usurped the action of IL-6 and TGFb to support increased lymphocyte infiltration [5]. One hypothesis is that synovial fibroblasts release membrane microvesicles (MV > 1µm) that bind to other cells, such as endothelial cells, and deliver signalling molecules or change the cell's adhesive surface [6,7]. Indeed increased levels of circulating MV in RA have been shown to be related to disease activity [8]. We now wish to characterise the MV derived from resolving and very early RA fibroblasts and assess their role in modulating endothelial and leukocyte behaviour. Identifying key fibroblast derived mediators responsible for the switch from resolving to persistent lymphocyte recruitment will enable the development of novel therapeutic agents that target fibroblasts to restore normal patterns of lymphocyte entry and exit. References 1. Filer A. The fibroblast as a therapeutic target in rheumatoid arthritis. Current Opinion in Pharmacology 2013;13:413-9. 2. Cush JJ. Early rheumatoid arthritis -- is there a window of opportunity? J Rheumatol Suppl 2007 Nov;80:1-7. 3. Raza K et al. Early rheumatoid arthritis is characterized by a distinct and transient 68 synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther 2005;7(4):R784-R795 4. McGettrick HM et al. Fibroblasts from different sites may promote or inhibit recruitment of flowing lymphocytes by endothelial cells. Eur J Immunol 39, 113-125, 2009. 5. McGettrick, HM et al. Fibroblasts lose their immunosuppressive ability early in the development of Rheumatoid Arthritis: Effects on lymphocyte recruitment. Annals of Rheumatic Diseases, 2014, 73s1:A19 doi: 10.1136/annrheumdis-2013-205124.43. 6. Loyer X et al. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res 2014;114:345-353. 7. Barteneva NS et al. Circulating microparticles: square the circle. BioMed Central Review 2013, 14;23. 8. Sellam J et al. Increased levels of circulating microparticles in primary Sjogren’s syndrome, systemic lupus erythematosus and rheumatoid arthritis and relation with disease activity. Arthritis Res Ther 2009, 11:R156. How are you planning to ensure adequate supervision? The student will be part of the Leukocyte Trafficking Group (www.birmingham.ac.uk/leukocyte-trafficking ~20 members ) based in the IBR West Extension, and the Rheumatology Research Group (~40 members) located within University Hospital Birmingham. In addition, the supervisors collaborate with Dr Paul Harrison, a world leading expert on MV biology and their analysis, based in the Centre for Translational Inflammation Research. Collectively, these have created a strong mentoring environment and well established training system which will support the student throughout their studies. The student will attend 3 separate weekly meetings dedicated to (i) stromal cell biology, (ii) leukocyte trafficking and (iii) Rheumatology. Supervisors, or existing lab members, have extensive experience in the specialist techniques, such as 3D multi-cellular flow-based adhesion assays, required for the project. Moreover, this project will run alongside research being undertaken as part of Dr Helen McGettrick’s Arthritis Research UK Career Development Fellowship. The student role. The student will test the hypothesis that the phenotype and function of MV release from synovial fibroblasts are different in acute and chronic inflammation. The main aims of the project will be to: 1. Characterise MV released from synovial fibroblasts isolated from patients attending BEACON. 2. Analyse the ability of fibroblast derived MV to bind to endothelial cells. 3. Using flow based adhesion assays, examine the bioactivity of fibroblast derived MV (i.e. the ability of fibroblast derived MV to effect endothelial recruitment of leukocytes). Key practical skills developed will include cell culture (aseptic technique), quantitative PCR, flow cytometry, confocal microscopy, flow-based adhesion assays. 69 Lead Supervisor: Prof. Jane McKeating Contact Email: Telephone: Co Supervisors: Dr. Alan Zhuang Project Title: Schools: Circadian rhythms and HIV infection. Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline The immune system is modulated by a wide variety of environmental influences and recent studies highlight a role for circadian rhythms to regulate host innate and adaptive immune responses via the expression of “clock genes”1,2. Macrophages show circadian responses to pathogen-associated challenges via clock genes such as Rev-erbA that regulate expression of cytokines IL6, CXCL6, CXCL11 and chemokine CCL23,4. Much of the published literature is based on animal models and there is a need to investigate the influence of circadian rhythms on innate immunity and susceptibility to infection in humans. Recent studies reporting that T cell activation in human immunodeficiency virus (HIV) infection associates with cortisol levels5 and that HIV-encoded Tat protein alters circadian activity6 suggest a role for clock genes in the viral lifecycle. Hypothesis: The cellular response to HIV infection is regulated by oscillations in clock gene expression. We will evaluate this hypothesis with the following objectives: 1. Does T cell circadian cycle associate with permissivity to support HIV replication? Experiments will utilize reporter HIV strains engineered to express GFP or luciferase, enabling rapid quantitation of viral replication. 2. Do changes in viral replication correlate with clock gene expression? 3. Does modulation of clock gene expression affect HIV infectivity or cytokine responses? These studies will increase our understanding of host pathways that regulate HIV replication and will influence HIV vaccine trial design. References 1. Curtis et al (2014). Circadian Clock protein and immunity. Immunity 40: 178-86. 2. Gibbs et al (2014). An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nat Med 20: 919-26. 3. Keller et al (2009). A circadian clock in macrophage controls inflammatory immune responses. Proc Natl Acad Sci USA 106:21407-21412. 4. Gibbs et al (2012) The nuclear receptor Rev-ErbA mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. PNAS USA 109:582-587. 5. Patterson et al (2013). Cortisol patterns are associated with T cell activation in HIV. PLOS ONE 8: e63429. 6. Wang, T. et al. (2014). HIV Tat protein affects circadian rhythmicity by interfering with the circadian system. HIV Med 15: 565-70. How are you planning to ensure adequate supervision? The student will be supervised by Prof McKeating and Dr Zhuang, providing constant advice and supervision during the course of the project. The student will have weekly 1-1 70 meetings with the supervisors to monitor progress, troubleshoot technical problems and provide advice and encouragement. The student role. This project offers the potential for training in key aspects of medical research including molecular virology and cell biology. The student would work alongside a team of scientists, working in novel areas of research with key relevance to medically important pathogens. The student will be trained to work with ACDP2 pathogens as well as a critical working knowledge of techniques such as tissue culture, virus replication, Western blot, transfections and immuno-fluroescence. Within the course of the placement it is expected the student would develop as an independent researcher able to design and conduct their own experiments; with the aim to acquire sufficient quality and novelty to merit publication. In addition the student would be expected to play an active part in the group including journal clubs and lab meetings. 71 Lead Supervisor: Jane McKeating Contact Email: Telephone: Co Supervisors: Dalan Bailey Project Title: Optimising lentiviral pseudotypes to study Ebola virus tropism. Schools: Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Ebola virus (EBOV) is a member of the Filoviridae family that causes severe hemorrhagic fever with up to 90% mortality. Unsurprisingly EBOV is classified as a bio-safety level 4 pathogen, limiting studies to designated laboratories with appropriate containment facilities. Lentiviral pseudotypes expressing foreign viral encoded glycoproteins have been used to study internalization pathways of many pathogenic human viruses including HIV and SARS under general laboratory conditions. Pseudotypic viruses undergo a single round of infection and expression of reporter proteins provides a quantitative assessment of glycoprotein-dependent particle entry. Recent reports demonstrate the infectivity of lentiviral pseudotypes expressing the EBOV glycoprotein GP1,2, validating this approach for studying viral tropism. EBOV GP1,2 regulates virus entry into cells and is a major virulence factor implicated in pathogenesis, including cytopathicity, endothelial dysfunction and immune suppression (1,2). GP1,2 expression is regulated by an RNA editing mechanism where full-length GP1,2 mRNA is produced by slippage of the viral polymerase at an editing site (3,4). Approximately 20% of transcripts are produced in this way with the remaining transcripts containing a premature stop codon which encodes a soluble truncated glycoprotein (sGP). A recent study reported that high levels of GP1,2 reduced the infectivity of lentiviral pseudotypes by undefined mechanisms (5). Previous studies highlight a role for GP1,2 in evading host immune responses leading us to suggest that the relative expression of GP1,2 and sGP regulate virus production, infectivity and evasion of innate and adaptive host immune responses. In this project we will modify the region of EBOV at which editing occurs to alter the rate of GP1,2 and sGP production and assess the impact on lentiviral pseudotype production and infectivity. These studies will optimise the production of EBOV pseudoparticles to study cellular tropism. References 1. Mohamadzadeh M, et al. 2007. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol 7:556-567. 2. Yang ZY, et al. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nature Medicine 6:886-889. 3. Sanchez A, et al. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. PNAS USA 93:3602-3607. 72 4. Volchkov VE, et al. 1995. GP mRNA of Ebola virus is edited by the Ebola virus polymerase and by T7 and vaccinia virus polymerases. Virology 214:421-430. 5. Mohan GS, et al. 2014. Less is more: Ebola surface glycoprotein expression levels regulate virus production and infectivity. J Virology, in press. How are you planning to ensure adequate supervision? The student will be co-supervised by members of the McKeating and Bailey groups. Our joint expertise in viral entry and cell-cell fusion mechanisms will ensure the prospective student is supervised to a high level, both technically and theoretically. The student will have regular (weekly) 1-1 meetings with the supervisors to monitor progress, troubleshoot technical problems and provide advice and encouragement. The student role. This project offers the potential for training in key aspects of medical research including molecular virology and cell biology. The student would work alongside a team of scientists, working in novel areas of research with key relevance to medically important pathogens. The student will be trained to work with ACDP2 pathogens as well as a critical working knowledge of techniques such as tissue culture, virus replication, Western blot, transfections and immuno-fluroescence. Within the course of the placement it is expected the student would develop as an independent researcher able to design and conduct their own experiments; with the aim to acquire sufficient quality and novelty to merit publication. In addition the student would be expected to play an active part in the group including journal clubs and lab meetings. 73 Lead Supervisor: Sally Roberts Contact Email: Telephone: Co Supervisor: s.roberts@bham.ac.uk Project Title: The role of hypoxia inducible factors in the human papilloma virus lifecycle and pathogenesis. Schools: Cancer Sciences and Immunity and Infection Jane McKeating Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Human papilloma virus (HPV) infections are associated with the development of over 300,000 epithelial cancers each year. Of the 275,000 women who die from cervical cancer, 80% occur in low-economic countries where the incidence of HPV-associated cancers is increasing. HPV prophylactic vaccines are not widely available in these countries and offer no therapeutic cure to individuals who have developed cancer. Thus, there is an urgent need for more affordable and easily administered anti-viral and therapeutic interventions. To do this we need a better understanding of the HPV life cycle and of mechanisms associated with HPV pathogenesis. HPV has evolved numerous strategies to hijack the host cell machinery to establish and maintain infection, including the stabilization of hypoxia inducible transcription factors (HIF)1. HIFs regulate cellular metabolism, angiogenesis, proliferation and migration, enabling a cell to respond to a low oxygen or hypoxic environment2. HPV and HIF-1α have been reported to synergistically promote cancer lesions in transgenic mice3. Furthermore, increased HIF expression correlates with a poor prognosis of patients with cervical cancer lesions. We will investigate whether: (1) HPV promotes HIF transcriptional activity at different stages of the HPV life cycle; (2) HPV stabilizes HIF in anogenital and oropharyngeal culture systems and (3) inhibiting HIF activity impacts on HPV genome replication and protein expression. This project will increase our understanding of the role HIFs play in the HPV lifecycle and whether they provide a new therapeutic target. References 1. Tang, et al. Overexpression of human papilloma type 16 oncoproteins enhances HIF protein accumulation and VEGF expression in human cervical carcinoma cells. (2007). Clin Cancer Research 13: 2568-2576. 2. Wilson, et al. Hypoxia inducible factors in liver disease and hepatocellular carcinoma: Current understanding and future directions. (2014). J Hepatol, in press. 3. Lu, et al. HIF-1 facilitates cervical cancer progression in HPV 16 transgenic mice. (2007). Am J Pathol 171: 667-681. How are you planning to ensure adequate supervision? The student will be co-supervised by members of the Roberts and McKeating groups. As such there will be constant advice and supervision during the course of the project from group leaders, their postdocs and PhD students. Our joint expertise in HPV biology (Roberts) and HIF biology (McKeating) will ensure the prospective 74 student is supervised to a high level, both technically and theoretically. The student will have regular (weekly) 1-1 meetings with the supervisors to monitor progress, troubleshoot technical problems and provide advice and encouragement. The student role. This project offers the potential for training in key aspects of medical research including molecular virology and cell biology. The student would work alongside a team of scientists, working in novel areas of research with key relevance to medically important pathogens. The student will be trained to work with ACDP2 pathogens as well as a critical working knowledge of techniques such as tissue culture, virus replication, Western blot, transfections and immuno-fluroescence. Within the course of the placement it is expected the student would develop as an independent researcher able to design and conduct their own experiments; with the aim to acquire sufficient quality and novelty to merit publication. In addition the student would be expected to play an active part in the group including journal clubs and lab meetings. 75 Lead Supervisor: Dr Mike Milward Contact Email: Telephone: M.R.Milward@bham.ac.uk 0121 466 5132 Co Supervisor: Prof Paul Cooper, Dr Will Palin Project Title: Novel non-antibiotic-based light irradiation approaches for decontamination Department: School of Dentistry Will the project require a Home Office working with animals licence? NO Is the Project Cancer related? NO Project Outline Increasing bacterial antibiotic resistance is causing major concern within the community and for healthcare professions and ultimately the World Health Organisation has identified antibiotic resistance as a major threat to public health. The Assistant Director-General for Health Security has stated that “Without urgent, coordinated action by many stakeholders, the world is headed for a post-antibiotic era, in which common infections and minor injuries which have been treatable for decades can once again kill”. It is therefore of major importance that alongside development of new types of antibiotics that other modalities to treat bacterial infections are developed. Photobiomodulation or Low level light therapy (LLLT) utilises low power (<500mW) light emitting diodes (LEDs) or lasers which have a direct biological effect. Varying the wavelength and dose (irradiance x time) of light delivered has been shown to provide a range of beneficial biological actions including increased healing, reduced inflammation and antibacterial effects. Addition of a photosensitizer can also be used to enhance the antimicrobial action of light – this approach is termed photodynamic therapy (PDT). A number of natural plant based extracts as well as synthetic nanoparticles have been shown to exhibit antimicrobial action when exposed to certain light wavelengths and irradiation conditions. Notably in general it is the generation of reactive oxygen species following the light activation of these intermediaries which results in the bactericidal activity. This project aims to investigate the potential for LLLT and PDT to inhibit bacterial growth and viability. Studies will investigate both direct action of light (LLLT) and via use of photosensitizing agents, such as natural plant-based extracts and synthetic nanoparticles (PDT). The ultimate aim is to develop a non-toxic, non-antibiotic based approach for decontaminating infected wounds throughout the body as well as related healthcare devices and equipment. The study will comprise 3 core research areas: 1. Light measurement: The initial objective is to accurately characterise the light sources to be used in this study. Research into LLLT often fails to appropriately and/or accurately characterise and report the light energy that cells receive, which undermines 76 the findings reported. Therefore initial studies will underpin the downstream understanding of the light delivered for use in LLLT & PDT using established methods in the research unit. 2. Bacterial Culture and Antibacterial assay: A range of Gram positive & negative bacteria associated with wound infections will be grown using in both broth and biofilm cultures. The growth characteristics will be determined using turbidity and colony counting assays. Data generated will identify optimal conditions for use in subsequent antibacterial assays.: Studies will subsequently determine the effect on bacterial cell growth and viability of (i) varying light irradiances/doses and wavelengths, and (ii) the use of a range of novel photosensitizing agents. 3. Cytotoxicity analysis: Light irradiation conditions identified as having antibacterial activity by direct action or via the photosensitizing agent intermediary will be investigated with regards to their application safety in eukaryotic cells in vitro. Standard metabolic and genotoxic assays, eg MTT and DNA comet, will be applied in this aspect of the work. It is envisaged that many irradiation parameters will be investigated as part of this work using a bespoke high-throughput light delivery device in order to determine the optimal wavelength and dose for decontamination. Combined this data will be used to underpin the development of a new therapeutic device for the disinfection of wounds as well as healthcare contaminated devices and equipment. References 1. Ultraviolet Radiation in Wound Care: Sterilization and Stimulation. Gupta A, Avci P, Dai T, Huang YY, Hamblin MR. Adv Wound Care (New Rochelle). 2013 Oct;2(8):422-437. 2. Novel strategies to fight Candida species infection. Rodrigues ME, Silva S, Azeredo J, Henriques M. Crit Rev Microbiol. 2014 Nov 10:1-13. 3. Ultra-low power laser stimulation impairs the adhesion of Staphylococcus aureus to primary human cells, and interferes with the expression of staphylococcal pathogenic factors. Petruzzelli S, Congiu A, Gallamini M, Pompei R. New Microbiol. 2014 Apr;37(2):193-9. Epub 2014 Apr 1. 4. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of Porphyromonas gingivalis. Nagahara A, Mitani A, Fukuda M, Yamamoto H, Tahara K, Morita I, Ting CC, Watanabe T, Fujimura T, Osawa K, Sato S, Takahashi S, Iwamura Y, Kuroyanagi T, Kawashima Y, Noguchi T. J Periodontal Res. 2013 Oct;48(5):591-9. 5. Flow cytometric assessment of Streptococcus mutans viability after exposure to blue light-activated curcumin. Manoil D, Filieri A, Gameiro C, Lange N, Schrenzel J, Wataha JC, Bouillaguet S. Photodiagnosis Photodyn Ther. 2014 Sep;11(3):372-9. 77 How are you planning to ensure adequate supervision? All the techniques proposed are well established within our research group, with staff and associated laboratory personnel experienced in undertaking these experiments. In addition we have a dedicated team of laboratory technicians who support students in initial laboratory induction/training and also provide the day-to-day bench support for experiments. Any student undertaking this project will have the necessary resource & support required to successfully complete their project. Regular meetings, likely weekly initially, will be organised to discuss progress and to provide guidance and further ad hoc meetings will occur. The supervisors have extensive experience in both undergraduate and postgraduate supervision. The student role. For students to get the most out of this project they will need to work diligently and show commitment to developing the necessary technical skills to undertake this study. Initially this will involve a thorough literature review to familiarise themselves with the underpinning published literature and attending training in the laboratory work. The student is always encouraged to discuss any project related issues with the supervisors who operate an ‘open door’ approach in their support. 78 Lead Supervisor: Professor Tim Mitchell Contact Email: Telephone: t.j.mitchell@bham.ac.uk Tel: 46779 Personal assistant : c.d.chapman@bham.ac.uk Co Supervisor: Dr Andrea Mitchell a.m.mitchell@bham.ac.uk Project Title: Evaluation of statins to reduce inflammation using in vitro models of pneumococcal pneumonia and meningitis Institute of Microbiology and Infection, School of Immunity and Infection, Biosciences Building Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Streptococcus pneumoniae (the pneumococcus) is part of the normal bacterial flora of humans but can also cause serious life threatening diseases such as pneumonia and meningiitis. We have shown that a pore forming toxin (pneumolysin) plays a major role in the pathology of these diseases. This toxin uses membrane cholesterol as its receptor. This project will therefore investigate whether the use of statins (which block cholesterol synthesis in mammalian cells) can reduce the effect of the toxin in disease pathology. In order to do this you will test the effect of statins (simvastatin) on the ability of the pneumococcus to induce pathology in cell culture models of blood brain barrier (human brain endothelial cells) and lung (alveolar basal epithelial cells). You will use several clinical isolates of the pneumococcus that differ in their ability to produce active toxin as well as bacterial mutants in which the gene for the toxin has been deleted or altered. The readouts will be measurement of baterial attachment and toxicity to the cell culture models. The effect of statins on cells will be monitored by measuring levels of cholesterol in the cell. The amount of inflammation induced by the bacteria or isolated toxin in the presence or absence of statins will be quantified by measuring cytokine profiles. Level of apoptosis will also be measured using standard assays. References Mitchell, T.J. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nat. Rev. Microbiol. 2003. 1 (3) 219-30. Boyd, A.R., Hinojosa, C.A., Rodriguez, P.J., Orihuela, C.J. Impact of oral simvastatin therapy on acute lung injury in mice during pneumococcal pneumonia. BMC Microbiology 2012, 12; 73. How are you planning to ensure adequate supervision? Day to day routine laboratory supervision(how to use equipment, grow cell lines etc) will be provided by the laboratory manager. Day to day scientific supervision will be provided by the named second supervisor or one of the post-doctoral fellows in the 79 laboratory. The student will have weekly meetings with Professor Mitchell to discuss project progress and plans. The student will also attend and present at the weekly laboratory meetings so that he/she is also aware of the other research projects running in the laboratory and what scientific techniques can be learned. The student role. The student will initially be given one-to-one supervision but will then be expected to gain independence in experimental design and take ownership of the project. The student will be expected to present data at regular group meetings and to interact with collaborators. Ideally the student will be expected to produce a publication at the end of the project. 80 Lead Supervisor: Dr Neil Morgan Contact Email: Telephone: n.v.morgan@bham.ac.uk 0121 414 6820 Co Supervisor: Mr Ben Johson Project Title: Molecular Genetic Investigation Platelet-based Bleeding Disorders Department: Clinical and Experimental Medicine of Inherited Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? Project Outline Background Platelets play an important role in normal haemostasis to prevent excess blood flow following vascular injury and can be regulated by aggregation, adhesion, secretion or procoagulant activities. Platelet bleeding disorders can present with variable penetrance ranging from mild to severe bleeding. For example Von Willebrand disease is the most common inherited bleeding disorder in which the mild forms are highly under diagnosed. Therefore there are a large number of patients with unclassified platelet bleeding disorders which underlies the need for comprehensive molecular diagnostic tools which will increase the capacity for early and rapid identification of these disorders. The recent advent of whole exome sequencing and next generation technologies has greatly enhanced the probability and speed of identifying mutations and hence causative genes in such conditions. Using this approach we have recently identified novel genes (ANKRD18A and SLFN14) in affected patients with inherited forms of thrombocytopenia (low platelet count) which causes severe bleeding. We have established a cohort of over 400 patients with platelet-based bleeding disorders. In this project, you will be trained in the use of next generation sequencing to identify genetic mutations in a small subgroup of these with a clear phenotype, and perform corresponding biochemical studies to verify the defect. Techniques to be used in the project Gene identification studies (genetic mapping, bioinformatic analysis, second generation sequencing), functional analysis of mutant gene products (protein expression analysis, Western blotting, cell localisation studies, analysis of downstream target genes). References 81 1. Daly ME, Leo VC, Lowe GC, Watson SP, Morgan NV (2014) What is the role of genetic testing in the investigation of patients with suspected platelet function disorders? Br J Haematol, 165, 193-203. 2. Stockley J*, Morgan NV*, Bem D, Lowe GC, Lordkipanidzé M, Dawood B, Simpson MA, Macfarlane K, Horner K, Leo VC, Talks K, Motwani J, Wilde JT, Collins PW, Makris M, Watson SP, Daly ME: UK Genotyping and Phenotyping of Platelets Study Group (2013) Enrichment of FLI1 and RUNX1 mutations in families with excessive bleeding and platelet dense granule secretion defects. Blood, 122, 4090-3. * equal contribution 3. Watson SP, Lowe GC, Lordkipanidzé M, Morgan NV; GAPP consortium (2013) Genotyping and phenotyping of platelet function disorders. J Thromb Haemost 11 (Suppl 1), 351-63. How are you planning to ensure adequate supervision? From a day to day basis both myself and Mr Johnson will be directly involved in the laboratory supervision of the student. The student role. The student will perform the techniques mentioned above as well as playing a role in defining the clinical phenotype of the inherited platelet-based bleeding disorders. The immediate focus of the planned project will be the identification of new causative genes using the powerful technique of whole exome sequencing. Further investigation of the role of the identified disease-causing mutations/genes will be performed. The precise techniques to be applied will depend on what type of gene is identified and what is already known, as well as the availability of relevant patient material such as cryopreserved peripheral blood mononuclear cells, disease tissue and/or cell lines. Functional analysis of human mutated genes could include cellular transfection and localisation by immunocytochemistry/flow cytometry, and in situ mutagenesis and expression of mutant proteins. Real-time PCR and transcriptional array analysis may be required to investigate expression of the wild type gene or, in the case of a transcription factor, to investigate the effect of mutations on regulatory activity. The project is highly likely to yield novel genes for these platelet-based bleeding disorders leading to a publication. 82 Lead Supervisor: Professor Paul Moss Contact Email: p.moss@bham.ac.uk Telephone: Co Supervisors: Miss Louise Hosie Dr Jianmin Zuo Project Title: ‘HLA-C restricted’ T cell immunotherapy for the prevention of cytomegalovirus disease after stem cell transplantation Department: School Of Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Previous clinical trials within our group have demonstrated the successful treatment of cytomegalovirus (CMV) disease within transplant (HSCT) patients involving the transfer of CMV-specific CD8+ T cells isolated from seropositive donors1. Despite these encouraging results, this therapy becomes difficult to apply to seronegative patients that have either undetectable or rare CMV-specific CD8+ T-cells to isolate2. In order to overcome this problem, an alternative approach is to transfer TCR specificity to transgenic, i.e. transfer known TCR specificities to primary T cells. Currently, adoptive therapy with HCMV-specific CD8+ T-cells TCR restricted through HLA-A2 alleles are successful; however this allele is only present in ~30% of the UK Caucasian population. Studies in our laboratory have recently identified a novel immunodominant HLA-Cw*0702 restricted CD8+ T-cell response which stimulates very strong T cell immune responses in healthy people (Hosie et al, unpublished data). This HLA-C allele is expressed at a much higher percentage within the human population, reaching a prevalence of 41%. We plan to generate HLA-C restricted CMV-specific CD8+ T cells using TCR transfer technology. These cells can then be used to treat CMV disease, especially for the CMV negative immunosuppressed HSCT recipient. 83 Experimental Plan:1. CD8+ T cell clones have been generated and will be purified with MHC class I tetramers. RNA will then be extracted and the TCR gene will be cloned, sequenced and subcloned into retrovirus expression vector. 2. The retrovirus expressing the TCR will then be produced through transfection and introduced into primary CD8+ T cells. The expression of the TCR will be confirmed by Flow Cytometry after staining with the HLA-peptide tetramer. 3. The TCR transfected T cell will be assessed for their ability to kill human cells which are infected with cytomegalovirus in the laboratory. This a prelude towards using these reagents in cellular immunotherapy protocols. The student will join one of the largest translational research units within the University. The group has successfully trained over 20 B.Med.Sci (Clinical Sciences) students in recent years. References 1 Paul Moss, and Alan Rickinson, 'Cellular Immunotherapy for Viral Infection after Hsc Transplantation', Nat Rev Immunol, 5 (2005), 9-20. 2 Andrea Schub, Ingrid G. Schuster, Wolfgang Hammerschmidt, and Andreas Moosmann, 'Cmv-Specific Tcr-Transgenic T Cells for Immunotherapy', The Journal of Immunology, 183 (2009), 6819-30. How are you planning to ensure adequate supervision? Miss Louise Hosie and Dr Zuo will be guiding the student about the project and meeting regularly for discussion. Miss Hosie will help the student to master the labbased technologies and also perform the day to day direct supervision. The student role. 1. In the lab, the student will carry out the lab work, such as PBMC separation, FACS staining and in vitro function assay of CD8+ T cells. 2. The student will attend the internal and external seminars of the department, will attend the group lab meetings and present their data, also will attend the journal club and discussion relevant paper to broad their scientific knowledge. 84 Lead Supervisor: Dr Ye Htun Oo Contact Email: Telephone: y.h.oo@bham.ac.uk MRC Clinician Scientist & Honorary Consultant Hepatologist Room 536, 5th Floor, IBR; Centre for Liver Research& NIHR BRU University of Birmingham & UHB NHS Foundation Trust Wolfson Drive, B15 2TH, Edgbaston, Birmingham, UK Co Supervisor: Mr Thomas Pinkney Dr Tariq Iqbal Project Title: Human hepatic innate lymphoid cells can be controlled by regulatory T cells Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Background Innate lymphoid cells (ILCs) are emerging as crucial effectors of innate immunity and tissue remodeling. ILCs are instrumental in immunity to invading microbes and also in mucosa immunity. ILC had been classified into Type1-3 depending on their surface markers, transcription factors and cytokine profile. The role of innate lymphoid cells in liver diseases in unexplored especially its regulation by regulatory T cells. Our recent preliminary data suggested the ILC are present in human inflammatory liver disease and inflammatory bowel diseases. Aim of the project To investigate the phenotypic of ILC subsets in human inflammatory liver disease and inflammatory bowel diseases. Project outline To investigate the ILC subsets, frequency in different human liver and bowel tissues by immunohistochemistry and confocal microscopy. Liver infiltrating ILC cells phenotype by analysing surface markers (including lineage markers, CD127, CD161. We then analyse its homing receptors, functional receptors, effector cytokines and transcription factors in explanted human liver tissues from diseased and normal livers/ hepatic resection. Same experiments will be performed with inflammatory bowel tissue. If time permits, we will co-culture ILC with regulatory T cells and assess its regulation by suppression of proliferation of ILC Benefits This will be the first study to investigate the role of ILC cells subsets in human liver tissue and inflammatory bowel diseases. We could apply this knowledge of 85 immunopathogenesis in the translational medicine to target specific ILC cells subsets to prevent inflammatory bowel diseases, cirrhosis, liver failure in future. Student will have a opportunity to sit in and learn liver diseases in autoimmune and viral hepatitis clinics and learn translational medicine and liver clinical trials. Student will have an opportunity to present the work in national and European meeting in results are novel. References Human type 1 innate lymphoid cells accumulate in inflamed mucosal tissues Jochem H Bernink1,10, Charlotte P Peters1,2,10, Marius Munneke3,4, Anje A te Velde1, Sybren L Meijer5, Kees Weijer4, Hulda S Hreggvidsdottir1,6, Sigrid E Heinsbroek1, Nicolas Legrand4,9, Christianne J Buskens7, Willem A Bemelman7, Jenny M Mjösberg1,8 & Hergen Spits Nature immunology How are you planning to ensure adequate supervision? We have chosen to combine the technical expertise of three supervisors to ensure the student is exposed to the maximal number of transferable research skills. Dr Ye Htun Oo is dedicated to 80% of his time in the laboratory, which is well equipped and set up for this type of study. Dr Iqbal and Mr Pinkey are both internationally renowned for their work in inflammatory bowel diseases. The Centre for Liver Research is part of the MRC Centre for Immune regulation and includes a large number of scientists working on liver immunology so the student will be working in a stimulating and supportive environment providing the student with exposure to research staff at all stages of their research careers. Appropriate training in research techniques and data analysis and presentation will be provided. We have weekly meetings with supervisors and interactions with other members of the research groups, who will be available day to day for advice and tuition. The student role. The student will be expected to perform experiments and conduct data analysis. They will present data to the supervisors and team and wider research groups and be expected to assimilate available published literature under guidance from the supervisors. They will be trained and expected to work using good laboratory practice under local standard operating procedures and be mindful of ethical use of human tissue specimens. They will be working in a large research teams and thus would be expected to interact with colleagues and use local facilities in a respectful manner. They could have an opportunity to sit in and observe in dedicated autoimmune hepatitis and viral hepatitis clinics to correlate laboratory bench work findings to pathogenesis and investigation of hepatic inflammation and autoimmunity. This would provide the student with an opportunity to link basic science to the patient. 86 Lead Supervisor: Dr. Jo Parish Contact Email: Telephone: j.l.parish@bham.ac.uk x58151 Co Supervisors: Dr. Gosia Wiench, Dr. Sally Roberts Project Title: Genome-wide analysis of CTCF binding distribution and HPV-induced reorganisation. Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Human papillomaviruses (HPVs) are the cause of benign and malignant lesions of the cutaneous and mucosal surfaces of the skin. High-risk HPV types (e.g. 16, 18, 31) are the cause of cervical cancer and many other anogenital carcinomas and cancers of the head and neck. To maintain persistent infection, papillomaviruses target several host cell pathways to alter cell cycle control and cellular gene expression creating a favourable environment for viral gene expression and genome replication1. Work in the Parish laboratory has shown that the host cell DNA binding factor CTCF associates with the genomes of low- and high-risk HPV types. The normal cellular function of CTCF is to regulate gene expression in multiple ways; CTCF binds to defined regions of the host cell genome and is required for maintaining epigenetic boundaries, gene enhancer blocking and regulating gene splicing and genetic imprinting. CTCF is able to contribute to these important mechanisms of host cell gene regulation by binding to DNA and either creating loop structures among cisregulatory elements or by physically blocking the path of the transcription machinery2. We have shown that CTCF recruitment to the HPV genome is important in the regulation of HPV gene regulation and splicing. We also have good evidence that HPV infection alters the normal cellular functions of CTCF leading to the hypothesis that recruitment of CTCF to the HPV genome alters CTCF distribution within the host cell genome and causes aberrant expression of host cell genes. To address this hypothesis, the specific aims of the project are as follows: 1. Determine the distribution of CTCF in the cellular genome before and after HPV infection. The genome-wide distribution of CTCF will be compared in primary human foreskin keratinocytes (HFKs) before and after transfection with HPV16 or HPV18 genomes. Chromatin immunoprecipitation followed by next generation sequencing (ChIP-Seq) will be used to determine the differences in CTCF distribution in this isogenic primary cell culture 87 system. 2. Determine whether HPV infection alters CTCF-dependent expression of candidate host genes. An alteration in CTCF binding at specific genomic loci could dramatically alter the activity of regional promoters and expression of imprinted genes. Regions that are identified to differentially bind CTCF following establishment of persistent HPV episomes will be analysed. The expression of genes within close proximity to these regions will be assessed by quantitative real-time PCR and western blot. Completion of these aims will provide important insight as to how an oncogenic virus manipulates host cell gene expression to enhance productivity of infection. Furthermore, identification of genes that are aberrantly expressed following HPV infection could enhance our understanding of how HPV causes cellular transformation and highlight possible biomarkers that could be used for early detection of HPV infection. References 1. Doorbar, J. (2005) The papillomavirus life cycle. J. Clin. Virol. 32(suppl):S7-15. 2. Phillips, J.E. and Corces V.G. (2009) CTCF: Master weaver of the genome. Cell. 137:11941211. How are you planning to ensure adequate supervision? The student will meet with the supervisors regularly to discuss progress and short and long-term plans. In addition, the student will attend and present at weekly lab meetings. Each week a member of the group presents their most recent experimental data, which is then discussed in an informal and supportive manner. We also have frequent journal club style meetings in which students and postdocs present and critique a recent publication. These meetings provide useful discussion points and an opportunity for feedback on presentation and critical analysis skills. In addition, the student will be informally supervised within the laboratory by an experienced postdoctoral research assistant. This provides a firm support network for students in the laboratory that can be adapted to an individual’s specific needs. The student role. The student will be trained in all necessary techniques and will therefore perform the laboratory work required to deliver the project goals. Importantly, to ensure ownership of the project, the student will be given every opportunity to contribute intellectually to the direction of the project, through discussion with the supervisors during weekly review meetings and during laboratory group meetings. To aid this intellectual input and to facilitate writing the thesis, the student will be expected to review the literature underpinning the project and to keep abreast of the current literature enabling the development and direction of the project and to ensure it remains cutting edge. 88 Lead Supervisor: Dr Eva Petermann Contact Email: Telephone: e.petermann@bham.ac.uk 41 49165 Co Supervisor: Dr Yavor Hadzhiev Project Title: DNA replication stress in the developing embryo Department: Cancer Sciences (Petermann) Clinical and Experimental Medicine (Hadzhiev) Will the project require a Home Office working with animals licence? Yes Is the Project Cancer related? No Project Outline DNA replication is the fundamental process by which all dividing cells copy their chromosomes. If progression of DNA replication forks is impaired, this can be a significant source of DNA damage, which in turn causes genomic instability or cell death. This is known as replication stress (1). A substantial number of gene that regulate DNA replication in human cells have been discovered (e.g. ATR, CHK1), and human patients with germ line mutations in these genes have been identified (2,3). Mutations in “DNA replication” genes cause autosomal recessive disorders including Seckel syndrome, Meier-Gorlin syndrome, microcephalic osteodysplastic primordial dwarfism, and others (4). Patients with these syndromes experience developmental defects, particularly proportional dwarfism and microcephaly. This suggests that replication stress during embryonic development may prevent organismal growth, especially that of the brain. This observation is fascinating because it suggests that control of DNA replication may be one of the fundamental mechanisms regulating human growth and development. However the roles of replication stress during development are not understood. Evidence that proper DNA replication underpins development is still lacking as data from in cell culture stduies have been inconclusive. What is really needed are animal models of the developing embryo, where conditions are very different from cells in culture (4). The aim of this project is to investigate whether deficiency in the gene mutated in ATR-Seckecl syndrome (ATR) causes replication stress and developmental defects in animal models of embryo development. Zebrafish embryos will be used as model system that allows us to study molecular processes including DNA replication as well as growth and development in vivo. You will use embryos that have a mutation in the ATR gene, and also treat wild type embryos with drugs that inhibit ATR or cause replication stress directly (e.g. hydroxyurea). You will then measure replication stress using DNA fibre analysis and DNA damage assays (5) and also monitor developmental defects and apoptosis, particularly in the brain. The main techniques to be used will be immunological and histological staining methods, microscopy, and Western blotting. This project will help us to understand how replication stress in the embryo is 89 connected to human growth disorders. Deciphering the molecular mechanisms underlying such disorders will be important for the development of treatments for growth defects. The project will be ideal for those who would like to gain experience in working with animal models and molecular biology (the molecular investigation of DNA and proteins). References 1. Zeman, M.K. & Cimprich, K.A. Causes and consequences of replication stress. Nature Cell Biology 16, 2-9 (2014). 2. Petermann, E. & Caldecott, K.W. Evidence that the ATR/Chk1 pathway maintains normal replication fork progression during unperturbed S phase. Cell Cycle 5, 2203-9 (2006). 3. O'Driscoll, M., Ruiz-Perez, V.L., Woods, C.G., Jeggo, P.A. & Goodship, J.A. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33, 497-501 (2003). 4. Klingseisen, A., & Jackson, A. P. Mechanisms and pathways of growth failure in primordial dwarfism. Genes & Development, 25, 2011–2024 (2011). 5. Jones, R. M., Kotsantis, P., Stewart, G. S., Groth, P., & Petermann, E. BRCA2 and RAD51 Promote Double-Strand Break Formation and Cell Death in Response to Gemcitabine. Molecular Cancer Therapeutics, 13, 2412–2421 (2014). How are you planning to ensure adequate supervision? The student will be supervised by Lead Supervisor Dr Petermann and Co-Supervisor Dr Hadzhiev, a postdoctoral researcher in the group of Dr Ferenc Muller. Day-to-day supervision will be ensured by Dr Petermann, who will introduce the student to the project, hold weekly meetings with the student and operate an open-door policy for the rest of the week. The student will further be supervised on a day-to-day basis by members of the Petermann lab, who have expertise in the scientific background and all molecular techniques used in this project. Dr Hadzhiev and members of the Muller lab will advise the student if and when needed with aspects relating to work with Zebrafish embryos. The Petermann lab holds weekly meetings were results are presented to the group and feedback obtained. The student will be encouraged to work on drafting the final thesis over the course of the project and regularly submit drafts to the Lead Supervisor to obtain feedback. Our group is part of a larger cluster of research groups with extensive expertise in human genetic disorders, DNA replication and DNA damage who hold weekly meetings and will provide further opportunities of support for the student. All previous project students in the Petermann lab have obtained first degrees on their projects. The student role. The student will be expected to familiarise themselves with the background and purpose of the project and the key literature in the field before and during the course of the project. With the help of the supervisor and group members, she/he will obtain a personal licence for Zebrafish and learn central laboratory methods in Zebrafish embryo culture and molecular biology of the DNA damage response. With support from the lab, the student will then apply the learnt methods to new experiments, and analyse and interpret the data obtained. She/he will keep a constant record of experiments conducted and results obtained, and regularly present the work to the group in lab meetings. The student is expected to develop increasing autonomy during the course of the project, take ownership of the project as much as possible, and be able to write a small thesis at the end. The student should spend all of the allocated time working on the project. The 90 student will be expected to work responsibly as part of a team, honour the rules of the lab, and immediately report any problems encountered to the supervisor. 91 Lead Supervisor: Laura JV Piddock Contact Email: Telephone: l.j.v.piddock@bham.ac.uk 0121 414 6966 www.antimicrobialagentsresearchgroup.com Mark Webber Co Supervisor: Project Title: From farm to fork: evaluating the dangers of antibiotic resistance in the food chain Department: Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No (Infection) Project Outline Bacteria resistant to antibiotics are an ever increasing threat to human health with pathogens for which no effective treatment remains being observed in hospitals around the world. Antibiotic resistance is a natural phenomenon and resistance genes and resistant strains can spread between environments and hosts. A number of pathogenic bacteria are common commensals or contaminants of food and the food chain is a significant reservoir of human infection. The use of antibiotics in farming has been implicated in the selection of resistant mutants which can then infect humans. Of particular concern is the emergence of resistance in animals to antibiotics used in humans as ‘last resort’ drugs for example colistin and carbapenems. Resistance to both these agents has been seen in bacteria from food animals. The aim of this project is to determine the level of resistance to critical human antibiotics in isolates of bacteria from food animals. Resistant isolates will be further analysed to identify the specific resistance genes and their immediate genetic context (i.e. are they on mobile elements which can be transferred between bacterial strains). Identification of antibiotic resistance genes in bacteria from animals will inform antibiotic use in animals, identify the emergence and prevalence of novel resistance mechanisms and ultimately help prevent the selection of mutant strains which pose a risk to human health. The project will contain a mix of clinical microbiology methods, antibiotic susceptibility testing, molecular biology, bacterial whole genome sequencing and bioinformatics. 1. 2. 3. References Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock, LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Micro. 2015 In Press. Cottell JL, Saw HT, Webber MA, Piddock LJ. Functional genomics to identify the factors contributing to successful persistence and global spread of an antibiotic resistance plasmid. BMC Microbiol. 2014 Jun 24;14:168. doi: 10.1186/1471-2180-14-168. Jiang HX, Song L, Liu J, Zhang XH, Ren YN, Zhang WH, Zhang JY, Liu YH, Webber MA, Ogbolu DO, Zeng ZL, Piddock LJ. Multiple transmissible genes encoding fluoroquinolone and third-generation cephalosporin resistance co- 92 located in non-typhoidal Salmonella isolated from food-producing animals in China. Int J Antimicrob Agents. 2014 Mar;43(3):242-7. doi: 10.1016/j.ijantimicag.2013.12.005. Epub 2013 Dec 17. How are you planning to ensure adequate supervision? Students will meet weekly with Prof Piddock and bi-weekly with both Prof Piddock and Dr Webber. They will be supervised daily by members of the antimicrobials research group which currently contains 15 full time members (www.antimicrobialagentsresearchgroup.com) ensuring appropriate cover will be available for student supervision. Piddock has successfully supervised numerous intercalating medical student projects to completion. The student role. The student will be responsible for performing and analysing experiments under the direction of Prof Piddock and Dr Webber and will be treated within the laboratory as any other member of the research team. Daily supervision will be by Dr Vito Ricci, a post-doctoral research fellow. This will ensure appropriate cover will be available for student supervision. Piddock and Webber have successfully supervised eight intercalating students in the last six years. The student will have responsibility for investigating the background of the project and developing a good awareness of the context and aims of the project 93 Lead Supervisor: Dr Myriam Chimen Contact Email: Telephone: m.chimen@bham.ac.uk 01214144042 Co Supervisor: Prof. Ed Rainger Project Title: Effect of pathway Department: School of Clinical and Experimental Medicine ageing on the adiponectin/PEPITEM Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline We recently identified a novel pathway that regulates the recruitment of T-lymphocytes across inflamed endothelium. This anti-inflammatory pathway involves adiponectin, an anti-inflammatory cytokine produced by the adipose tissue [1], known to modulate leukocyte recruitment in vivo [2]. In our laboratory, we have shown that adiponectin inhibits T-lymphocyte migration across inflamed endothelium. Adiponectin does not directly target T-lymphocytes; rather it stimulates B-lymphocytes to secrete a novel endogenous peptide (PEPITEM) that in turn modulates T-lymphocyte recruitment (Figure 1). This novel regulatory peptide stimulates the release of sphingosine-1-phosphate (S1P) by the endothelium that in turn modulates T-lymphocyte recruitment. Interestingly, the adiponectin/PEPITEM pathway is altered in autoimmune and chronic inflammatory diseases such as type 1 diabetes and rheumatoid arthritis (RA) and is therefore an exciting potential therapeutic target. Interestingly, our preliminary data indicate that the adiponectin/PEPITEM pathway is altered in the elderly which implies that there is a natural process of senescence in this pathway that could contribute to the risk of developing diseases such as RA. This project will overall aim to determine the effets of age on the adiponectin/PEPITEM pathway. 1 Lymphocyte rolling 2 β1/β2-integrin activation 4 3 6 Lymphocyte Lymphocyte spreading arrest and migration 8 Adiponectin 5 PEPITEM T-lymphocyte AdipoR1/2 CXCR3 9 DP2 S1PR1/4 T 7 B-lymphocyte Spsn2 CXCL9-11) Endothelial Cell COX PGD2 AA S1P SPHK1 B PEPITEM SPHK1 receptor Endothelial cell New steps in the regulation of Lymphocyte migration Figure 1: Schematic representation of the endogenous B-cell 94 mediated regulation of T-cell migration during inflammation. PEPITEM secretion from B-cells. PEPITEM signalling through an unknown receptor on the endothelium results in S1P production, which subsequently inhibits T-cell migration. (AdipoR1/2: adiponectin receptors 1 and 2; S1P: sphingosine-1-phosphate; S1P1/4: S1P receptors 1 and 4). Plan of investigation: Aim 1: Quantification of Adiponectin receptors in healthy ageing cohorts. We have measured the expression of both adiponectin receptors (AdipoR1 and AdipoR2) on healthy controls from a small cohort of subjects and found a negative correlation between both receptors expression and age. Our aim in this study is to quantify the expression of both receptors on defined age cohorts of 20-30 years old, 30 to 40 years old, 40 to 50 years old and 60 plus. In addition, the subjects will be grouped according to gender and ethnicity. The expression of both adiponectin receptors will be measured by flow cytometry and real-time-PCR following well-established protocols in our laboratory. Aim 2: Measure the effests on adiponectin and PEPITEM on lymphocyte transmigration in the healthy ageing cohort. In our T1D and RA study, the levels of adiponectin receptors on Blymphocytes negatively correlates with the inhibitory effects of adiponectin on peripheral blood lymphocyte transmigration. In this project we would like to assess the response of lymphocyte to adiponectin in a functionnal transmigration assay and correlate this with age and levels of adiponectin receptors on B-lymphocytes. References 1. Kadowaki T et al. (2006) Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J Clin Invest. 116:17841792. 2. Ouedraogo R et al. (2007) Adiponectin deficiency increases leukocyteendothelium interactions via upregulation of endothelial cell adhesion molecules in vivo. J Clin Invest. 117:1718-1726. How are you planning to ensure adequate supervision? The student will join the well-established collaborative research group of Prof. Rainger within the Institute for Biomedical Research. The student will collaborate with the group of Prof. Janel Lord in order to obtain the different age cohort samples. Within these groups, the student will attend meetings on a weekly basis with the core of people working on the PEPITEM project. 95 These meetings will allow informal and supportive discussion of the student’s progress and establishment of short and long term plans. The student will be supervised in the laboratory on a daily basis by an experienced post-doctoral fellow who conducted her PhD on PEPITEM (Dr Myriam Chimen). The student role. The student will learn a variety of useful and cutting-edge techniques including immune cell isolation and culture, culture of endothelial cells, flow cytometry, real-time PCR.The student will be involved in in-vitro work with human tissue only. The student will be required to read around the subject, present and participate in lab meetings and will be in a supportive laboratory atmosphere. The student will be responsible for analysing the data and keep detailed record of the work accomplished. Upon initial training, the student will be able to gain independence in the lab, contribute to experimental design and present the data in an appropriate manner. The student will have many opportunities to interact with lab members who will kindly offer support at a daily basis. 96 Lead Supervisor: Sally Roberts Contact Email: Telephone: 0121 414 7459 Co Supervisor: Joanna Parish Project Title: Mapping viral transcription profiles in life cycle models of oncogenic human papillomaviruses Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? YES Project Outline Oncogenic human papillomaviruses (HPV) infect the squamous epithelia lining the anogenital and oropharyngeal tracts and these infections are at risk of progressing to cancer. The introduction of prophylactic vaccines to protect against infection with the most prevalent HPV genotypes HPV16 and 18 is effective at reducing high-grade cervical premalignant disease. However, the bulk of cervical disease occurs in countries where it is difficult to introduce an expensive and cold-dependent vaccine. Furthermore, the incidence of HPV-induced oropharyngeal cancers are increasing at an alarming rate, primarily in men who are not offered the vaccine. Thus, this cancer-causing virus remains a significant threat to the human health and there is an urgent demand for cheap anti-viral/therapeutic interventions. The HPV life cycle is intimately linked to the differentiation of the host cell - the keratinocyte, such that new progeny are only produced in the most differentiated cells. This is achieved through a strict coordination of viral gene expression. We have modelled the life cycle of HPV16 and HPV18 in anogenital and oropharyngeal human keratinocytes. Upon growth of the HPV genome-containing cells in three dimensional organotypic raft culture we observe signficant differences in the execution of the life cycle in cells derived from different body sites. We hypothezie that these variations explain the different pathogenicities of HPV infection at these two sites. To gain insight into HPV infection at different body sites, the project aims to investigate the transcription profiles of HPV16 and HPV18 in anogenital and oropharyngeal keratinocytes. The project will use RT-PCR in combination with laser capture microdissection to define the spatial and temporal profiles of HPV transcripts in organotypic raft cultures. It is anticipated that this approach will identify major differences in the HPV16 and 18 life cycles in different target tissues which will help explain the natural history of cancer development in different epithelia. References Doorbar et al., 2012 The biology and life-cycle of human papillomaviruses. Vaccine 30S F55-70. How are you planning to ensure adequate supervision? The student will be embedded in the groups of Parish and Roberts and will benefit from the joint expertise of these two groups in HPV molecular and cell biology. The 97 student will meet weekly with the two supervisors to discuss progress and future direction. They will also participate in the combined weekly group meetings where the student will have the opportunity to present their data to an interested and knowledgeable group and where they will benefit from constructive feedback. The student role. The student will be exposed to HPV molecular and cell biology. They will be trained in all necessary techniques including primary epithelial cell culture, organotypic raft culture, RNA purification, RT-PCR and laser capture microdissection and will therefore perform the laboratory work required to deliver the project goals. Importantly, to ensure ownership of the project, the student will be given every opportunity to contribute intellectually to the direction of the project. To aid this intellectual input and to facilitate writing the thesis, the student will be expected to review the literature underpinning the project, enabling the development and direction of the project and ensuring it remains cutting edge. 98 Lead Supervisor: Dr Claire Shannon-Lowe Contact Email: Telephone: C.ShannonLowe@bham.ac.uk Co Supervisor: Dr Doug Ward Project Title: Identification of critical B cell and epithelial cell factors involved in entry of Epstein Barr virus. Department: Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Epstein Barr virus (EBV) is a human herpesviruses which infects approximately 90% of the human population and maintains a lifelong latent infection in the infected host. Importantly, EBV was also the first human tumour virus to be identified, and is associated with several cancers from different cell lineages including B-, T- and natural killer lymphocytes and epithelail cells. During the natural life cycle of the virus, EBV infects B lymphocytes and epithelial cells, and thanks to recombinant virus technology we have been able to identify which viral proteins are absolutely essential for infection of these two cell types. This is of critical importance when designing a vaccine. Entry into B lymphocytes requires the specific interaction between the viral glycoproteins gp350 and gp42 with the B cell CD21 and HLA class II respectively. In contrast, gp350 and gp42 are not essential for viral entry into epithelial cells. This is alternately mediated by the viral gH/gL interaction with various epithelial cell integrins (1-3). The interaction partner of one essential viral glycoprotein however remains elusive. Glycoprotein B is the viral protein which initiates the fusion between the viral envelope and both the B cell and epithelial cell plasma membranes, enabling delivery of the viral capsid inside the target cell (4). Recombinant viruses with the gB gene deleted cannot infect either cell type. In this project, the student will investigate which proteins present on the B cell and epithelial cell surface interact with the viral glycoprotein gB. The student will use both recombinant wild-type and gB-deleted viruses as well as purified multimerised gB to interact with the cell surface. We will use co-immunoprecipitation and proteomics to identify potential binding partners and immunoassays to confirm the protein-protein interactions. The student will be trained in various research techniques including primary cell culture, virological techniques, co-IP and Western blotting. 1. 2. 3. 4. References Shannon-Lowe et al., 2006. PNAS, 103(18):7065-70. Shannon-Lowe and Rowe, 2014. Curr Opin Virol, 78-84. Shannon-Lowe and Rowe, 2011. PLoS Pathogens, 7(5):e1001338. Neuhierl et al., 2009. J Virol, 83(9):4616-23. How are you planning to ensure adequate supervision? 99 This project will be supervised by Dr Claire Shannon-Lowe who will teach the student the techniques necessary for the project. Dr Shannon-Lowe will be available to meet on a daily basis to plan and discuss work. Dr Shannon-Lowe has expertise in the entry of EBV into B cells and epithelial cells and associated virological techniques. Dr Ward is an expert in proteomics and associated techniques and will be available to meet and give advice when necessary. The student role. The student will work alongside Dr Shannon-Lowe, Dr Ward and members of the B cell group. The project will provide the student with training in basic laboratory techniques which will not only provide a good basic understanding of how techniques are performed, but will enable the student to design and appropriately control experiments and correctly interpret the results. Laboratory techniques involved in this project include isolation and purification of lymphocyte subsets, cell culture, production of recombinant viruses, proteomics, co-immunoprecipitation and Western blotting. The student will also have the opportunity to discuss their work regularly at group meetings and will attend group journal clubs, seminars by members of Cancer Sciences and by visiting international researchers. 100 Lead Supervisor: Dr Adnan Sharif, Consultant Nephrologist. Contact Email: Telephone: Adnan.sharif@uhb.nhs.uk Co Supervisor: Andrew Ready, Consultant Transplant Surgeon. Jay Nath, Surgical Research Fellow & PhD Student. Project Title: Analysis of modifiable and non modifiable risk factors to predict renal transplant outcome. Department: Department of renal transplantation, University Hospital Birmingham NHS Foundation Trust. Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Renal transplantation remains the optimal treatment for patients with end stage renal failure conferring survival and quality of life advantages (1). Such are the benefits of renal transplantation that a greater number of patients are now offered this treatment, including those with complex medical problems as even in high risk patients survival is superior to remaining on dialysis. There has been a considerable change to both the donor and recipient transplant population over the past decade. Many these are reflective of the change in general population trends such as increasing age and BMI. However further immunological complexity has been observed with a greater proportion of highly sensitised patients, often from multiple previous failed transplants. Despite the ever-growing demand, transplantation is limited by the paucity of available organs and great efforts have been made to expand the donor pool over the past decade (2). These include utilisation of more high risk extended criteria donors, Donation after Circulatory Death kidneys, living kidney donation, anatomically complex organs and transplantation across ABO blood groups. Encouragingly, despite the increasing complexity of both donor and recipient factors, the outcome following kidney transplantation continues to improve with current 1 year graft and patient survival rates at over 90% (3). Most of the identified risk factors to predict transplant outcome (type of donor, immunological compatibility) has been derived from the American dataset (UNOS). However many of these population based findings are historical and have not been validated on a large second population. Through NHSBT (NHS Blood and Transplant) we have research permission to access the national renal transplant database containing over 22,000 renal transplant patients between 2001-2014. To our knowledge this is the largest and most complete dataset other than the UNOS registry, containing comprehensive donor and transplant information as well as both patient and graft survival information. The aim of this project would be analyse this dataset to identify risk factors that govern renal transplant outcome. We are particularly interested in the outcome amongst South Asian transplant recipients as this has been an underpublished area of research. Results and risk factors amongst high risk groups such as ABO 101 bloodgroup incompatible (ABOi) transplants, Multiorgan transplant recipients (eg combined liver kidney) and those with multiple previous failed transplants are also of great interest. The student will be based in the QE hospital Birmingham as part of an active clinical research team. They will be performing an interesting and highly productive role in analyzing a large national dataset. This project would be particularly suited to an aspiring academic clinician, ideally with an aptitude and interest in statistics. This position is heavily orientated towards publication and we would expect at least two publications from any BMedSc student, one of which should be as first author. Access to such a dataset in combination with the clinical and statistical knowledge within the trust make this an exciting and potentially highly lucrative opportunity. References 1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of first cadaveric transplant. N Engl J Med 1999;341:1725-30. 2. Nagaraja P, Roberts GW, Stephens M, Horvath S, Kaposztas Z, et al. (2014) Impact of Expanded Criteria Variables on Outcomes of Kidney Transplantation from Donors After Cardiac Death. Transplantation. 3. http://www.organdonation.nhs.uk/statistics/transplant_activity_report/current _activity_reports/ukt/survival_rates.pdf How are you planning to ensure adequate supervision? The student will receive a departmental induction and set clear objectives by the supervisors. Initial work will include a literature review to familiarise the student with current evidence in renal transplantation. They will work under the close supervision of the current final year PhD student who will oversee day to day training. Statistical guidance will be provided by the clinical team and the trust statistician (Dr Hodson). The student will be handling large amounts of patient sensitive data and will be given training in data protection. We will provide in house training on scientific writing and manuscript preparation. The student role. The student will be expected to act professionally and maintain ethical standards to protect patient sensitive data. In time they will be expected to become familiar statistical concepts and with the SPSS statistical programme. We would expect the student to be highly motivated and aim towards producing high quality pieces of work leading to publication. This project represents a significant opportunity for the aspiring academic. The student will be expected to attend and present their findings at the in house renal audit meetings. 102 Lead Supervisor: Shishir Shetty Contact Email: Telephone: s.shetty@bham.ac.uk 07879691053 Co Supervisor: Fedor Berditchevski, Christopher Weston Project Title: The role of CD151 in leucocyte recruitment to the human liver and hepatocellular cancer Department: Centre for Liver Research, Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Liver disease is dramatically increasing in the UK and globally. New medical therapies are urgently required to reduce the burden on liver transplantation. Inflammatory liver diseases are caused by a variety of factors but they all progress through a common pathway. This involves leucocyte recruitment from the circulation into liver tissue which drives tissue injury, fibrosis and can progress to cirrhosis. Leucocyte recruitment to the liver occurs within the low shear channels of the hepatic sinusoids where leucocytes interact with specialized sinusoidal endothelial cells (1). These channels are a unique environment for recruitment, they lack conventional adhesion molecules such as selectins and recruitment is mediated by atypical adhesion molecules such as vascular adhesion protein-1 (VAP-1) and the common lymphatic and vascular endothelial receptor- 1 (CLEVER-1) (2,3,4). Recently, using confocal microscopy, we have also demonstrated that leucocyte transmigration across human sinusoidal endothelial cells (HSEC) occurs via the conventional paracellular route and a novel transcellular route (3) Figure 1. Figure 1: (A) Confocal imaging of fluorescently stained lymphocytes transmigrating across monolayers of HSEC with demonstration of (B) paracellular and (C) transcellular migration. Intercellular interactions play a fundamental role during transendothelial migration. Assembly and reorganisation of cell-cell junctions are controlled at multiple levels and require tight co-ordination of various signalling pathways. The regulators of this process in the liver sinusoids are poorly understood and identifying key players in this process could lead to new treatments for inflammatory liver disease and liver cancer. Tetraspanin CD151 is a member of the family of four-transmembrane proteins (5). At the cellular level CD151 is known to interact with and regulates function 103 of laminin-binding integrins (adhesion, migration and invasion) (6). Along with several other tetraspanins CD151 was found at the cell-cell junctions and described as a regulator of homotypic intercellular interactions in epithelial and endothelial cells. In preliminary work we discovered that depletion of tetraspanin CD151 altered the cellular distribution and expression levels of ZO-1, a key structural component of intercellular junctions in epithelial and endothelial cells. We have also demonstrated that CD151 is expressed in diseased human liver tissue and in primary human liver sinusoidal endothelial cells (Figure 2). Figure 2: (A) Lysates from control (CD151+) and CD151-depleted cells (CD151-) were probed with specific antibodies. Re-expression of the wild type protein but not CD151 mutant which is unable to bind integrins in CD151- cells (CD151rec and CD151QRD cells, respectively), restored the cellular level of ZO-1. (B) CD151 expression in inflamed human liver tissue by immunohistochemistry and (C,D) ZO-1 and CD151 expression by immunofluorescence in isolated human HSEC. Aims 1) Study the expression of CD151 in normal and chronically inflamed human liver tissue and primary liver cancer. Use dual colour immunohistochemistry to confirm the cell specific expression of CD151. 2) Study the regulation of CD151 expression in primary human liver endothelial cells by using cytokine stimulation, co-culture with other liver cell populations and mediators of fibrosis and hypoxia. 3) The functional role of CD151 in leucocyte trafficking to the liver using in vitro flow adhesion assays with a combination of phase contrast and confocal microscopy and lentiviral knockdown of CD151 in primary human liver endothelial cells and tumour endothelial cells. References 104 1) 2) 3) 4) 5) 6) Shetty S, Toxicology Dec 30;254(3):136-46. (2008) Lalor P. J Immunol 169(2), 983-92 (2002) Shetty S. J. Immunol 186(7), 4147-4155 (2011) Shetty S Hepatology 56(4):1521-31 (2012) Tsujino, K. Am. J. Respir. Crit Care Med. 186, 170-180 (2012) Detchokul, S Jun 3 epub.Br. J. Pharmacol. (2013) How are you planning to ensure adequate supervision? This project involves three supervisors, Dr Shetty and Dr Weston are based in the centre of liver research in the medical school. They will provide guidance and supervision for studying liver tissue and isolating liver cells, furthermore they can directly supervise in the use of flow adhesion assays and confocal microscopy. Dr Berditchevski is based in Cancer Sciences and he will be able to supervise the student in transfection techniques and protein analysis. All three supervisors will liaise regularly to ensure that the student is gaining adequate training and guidance to complete the project. The student role. The student will be mainly based in the centre for liver research where they will be working both on liver tissue sections and isolated liver cells which are available through the liver transplant programme at the Queen Elizabeth Hospital Birmingham. All the protocols and techniques are established in the department- and the student will initially be taught these techniques followed by focussing on the molecule CD151. The role of CD151 in liver disease is poorly understood and there is limited literature on this subject. Using established techniques to study the role of CD151 will allow the student to gather novel data for publication. The student will also spend a part of their time in cancer studies with Dr Berditchevski. Dr Berditchevski is an expert on CD151 and has several important reagents and techniques which the student can use to study CD151 regulation and trafficking. The experiments in cancer studies will complement and support the work performed in the centre for liver research. 105 Lead Supervisor: Attila Sik Contact Email: Telephone: a.sik@bham.ac.uk 46018 Co Supervisor: Ferenc Muller f.muller@bham.ac.uk Project Title: Development of high throughput electrocardiogram recording of zebrafish embryos for drug screening Department: School of Clinical and Experimental Medicine Will the project require a Home Office working with animals licence? Yes Is the Project Cancer related? No Project Outline Effective chemical compound screening is of paramount importance for safe cardiac drug development. A difficult issue relates to the risk of serious adverse events that may only be discovered at a late stage in the adoption of new drug, such as idiosyncratic events or toxic effects that are difficult to identify and predict from preclinical development programmes. The vast majority of drug candidates fail during various test phases because of side or toxic effects. Using mammals for preliminary screening is expensive, slow and requires enormous numbers of animals. Alternatively, zebrafish embryos can be used because the electro cardiogram (ECG) is similar to mammals, a minimal amount of chemicals are necessary for drug testing, and embryo production is fast and inexpensive. Video-recording of zebrafish embryos’ heart activity is currently used for drug screening, but unlike ECG recording it lacks the temporal and dynamic resolution necessary for cardiac cycle component analysis. By combining expertise from electrical engineering, genetics, physiology and neuroscience, we are developing a method to perform high throughput recording from the heart of ~100 zebrafish embryos. Multichannel electrodes will register ECG signals under control conditions and after drug treatment for automatic analysis. The high throughput screening of drugs will potentially become an essential tool in cardiovascular research in the near future. References S.S.Dhillon, E.Doro, I.Magyary, S.Eggington, A.Sík and F.Müller (2013) Optimisation of embryonic and larval ECG measurement in zebrafish for quantifying the effect of QT prolonging drugs Plos One, 8(4):e60552 How are you planning to ensure adequate supervision? The student have access to both supervisors in a daily basis. Focused lab meeting is held every week to discuss the project where everyone involved in the project participate. The student role. 106 The student will perform single and multichannel recording from zebrafish heart, actively involved in electrode design, analyse data and test drugs for side and toxic effects. 107 Lead Supervisor: Alex Sinclair Head of the CSF Disorders Research Group, Neurobiology, School of Clinical and Experimental Medicine Contact Email: Telephone: a.b.sinclair@bham.ac.uk Co Supervisor: Wiebke Arlt Professor of Medicine, Head, Centre for Endocrinology, Diabetes and Metabolism (CEDAM) Project Title: Characterising a novel relationship between idiopathic intracranial hypertension and polycystic ovarian syndrome Department: Neurobiology & Centre for Endocrinology, Diabetes and Metabolism College of Medical and Dental Sciences Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? No Project Outline Idiopathic intracranial hypertension (IIH) is a condition of unknown aetiology characterised by elevated intracranial pressure (ICP) (Mollan, Markey et al. 2014). The condition causes chronic disabling headaches and papilloedema which results in visual loss in up to 25% of cases. Of particular interest is that the condition occurs almost exclusively (>90%) in obese women of childbearing age (Corbett, Savino et al. 1982). Intracranial pressure regulation is dependent on the balance between CSF production at the choroid plexus and drainage at the arachnoid granulation tissue but the underlying pathogenesis leading to disordered ICP in IIH is not known. A number of studies have highlighted the increased incidence of polycystic ovarian syndrome (PCOS) amongst IIH patients (39 – 57% of patients with IIH having PCOS, whilst the background risk of PCOS in the general population is 7-15% (Glueck, Iyengar et al. 2003, Tsilchorozidou, Overton et al. 2004, Glueck, Aregawi et al. 2005). IIH and polycystic ovarian syndrome (PCOS) share number phenotypic characteristics including female gender, obesity, and often hirsutism. PCOS is a heterogeneous condition, comprising clinical and biochemical hyperandrogenism, oligo/amenorrhoea and metabolic syndrome (Tsilchorozidou, Overton et al. 2004). 5-alphα reductase functions as a cortisol inactivating enzyme and it also plays a key role in activating testosterone into 5-alphα dihydrotestosterone (Bruchovsky and Wilson 1968). A significant body of evidence is accumulating suggesting dysregulation of 5αlpha reductase activity in PCOS: Increased 5-αlpha reductase activity has been demonstrated in urinary metabolites in PCOS (Stewart, Shackleton et al. 1990, Chin, Shackleton et al. 2000, Fassnacht, Schlenz et al. 2003, Tsilchorozidou, Honour et al. 2003, Vassiliadi, Barber et al. 2009) We suggest that the strong relationship between IIH and obese female patients strongly suggests that a neuroendocrine dysfunction, potentially 108 through dysregulation of androgen pathways, may play an important aetiological role. Hypothesis: Androgen dysregulation is involved in the aetiology of IIH AIM: 1. Characterisation of the key elements of the androgen signalling pathway in the choroid plexus. 2. Evaluate the effects of exogenous androgens (including androgen metabolites and regulating enzymes) on cerebrospinal fluid secretion (CSF) using a primary choroid plexus in vitro model. 3. Characterise the serum androgens and urinary androgen metabolites in IIH patients and compared to obese controls and PCOS patients. Methods: 1. Key elements of the androgen signalling pathway will be examined in the choroid plexus from brain sections, choroid plexus explants and primary choroid plexus epithelial cells (human and porcine) (Haselbach, Wegener et al. 2001): gene expression will be investigated by qPCR will be used to assess the expression and further protein localisation will be conducted by immunohistochemistry. 2. The effects of exogenous androgens on cerebrospinal fluid secretion (CSF) will be examined on a functional primary porcine choroid plexus in vitro model currently established in house: CSF secretion will be assessed using: 1) a fluorescent dextran assay; 2), by measuring Na+,K+-ATPase activity and 3) by Real Time PCR. Briefly, the fluorescent dextran will be added to both the apical and basolateral chambers and the change in the concentration of dextran in each chamber will be measured over a period of time. Na+,K+,ATPase activity will be assessed by measuring the amount of inorganic phosphate produced by the cells. 3. Using previously collected samples from IIH and control subjects, serum androgens will be evaluated by tandem mass spectrometry and 24-hour urine androgen excretion by gas chromatography/mass spectrometry. Data will be analysed and critically evaluated.(O'Reilly, Taylor et al. 2014) Key References 109 Bruchovsky, N. and J. D. Wilson (1968). "The conversion of testosterone to 5alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro." J Biol Chem 243(8): 2012-2021. Chin, D., C. Shackleton, V. K. Prasad, B. Kohn, R. David, J. ImperatoMcGinley, H. Cohen, D. J. McMahon and S. E. Oberfield (2000). "Increased 5alpha-reductase and normal 11beta-hydroxysteroid dehydrogenase metabolism of C19 and C21 steroids in a young population with polycystic ovarian syndrome." J Pediatr Endocrinol Metab 13(3): 253-259. Corbett, J. J., P. J. Savino, H. S. Thompson, T. Kansu, N. J. Schatz, L. S. Orr and D. Hopson (1982). "Visual loss in pseudotumor cerebri. Follow-up of 57 patients from five to 41 years and a profile of 14 patients with permanent severe visual loss." Arch Neurol 39(8): 461-474. Fassnacht, M., N. Schlenz, S. B. Schneider, S. A. Wudy, B. Allolio and W. Arlt (2003). "Beyond adrenal and ovarian androgen generation: Increased peripheral 5 alpha-reductase activity in women with polycystic ovary syndrome." J Clin Endocrinol Metab 88(6): 2760-2766. Glueck, C. J., D. Aregawi, N. Goldenberg, K. C. Golnik, L. Sieve and P. Wang (2005). "Idiopathic intracranial hypertension, polycystic-ovary syndrome, and thrombophilia." J Lab Clin Med 145(2): 72-82. Glueck, C. J., S. Iyengar, N. Goldenberg, L. S. Smith and P. Wang (2003). "Idiopathic intracranial hypertension: associations with coagulation disorders and polycystic-ovary syndrome." J Lab Clin Med 142(1): 35-45. Haselbach, M., J. Wegener, S. Decker, C. Engelbertz and H. J. Galla (2001). "Porcine Choroid plexus epithelial cells in culture: regulation of barrier properties and transport processes." Microsc Res Tech 52(1): 137-152. Mollan, S. P., K. A. Markey, J. D. Benzimra, A. Jacks, T. D. Matthews, M. A. Burdon and A. J. Sinclair (2014). "A practical approach to, diagnosis, assessment and management of idiopathic intracranial hypertension." Pract Neurol 14(6): 380-390. O'Reilly, M. W., A. E. Taylor, N. J. Crabtree, B. A. Hughes, F. Capper, R. K. Crowley, P. M. Stewart, J. W. Tomlinson and W. Arlt (2014). "Hyperandrogenemia predicts metabolic phenotype in polycystic ovary syndrome: the utility of serum androstenedione." J Clin Endocrinol Metab 99(3): 1027-1036. Stewart, P. M., C. H. Shackleton, G. H. Beastall and C. R. Edwards (1990). "5 alpha-reductase activity in polycystic ovary syndrome." Lancet 335(8687): 431-433. Tsilchorozidou, T., J. W. Honour and G. S. Conway (2003). "Altered cortisol metabolism in polycystic ovary syndrome: insulin enhances 5alpha-reduction but not the elevated adrenal steroid production rates." J Clin Endocrinol Metab 88(12): 5907-5913. Tsilchorozidou, T., C. Overton and G. S. Conway (2004). "The pathophysiology of polycystic ovary syndrome." Clin Endocrinol (Oxf) 60(1): 1-17. Vassiliadi, D. A., T. M. Barber, B. A. Hughes, M. I. McCarthy, J. A. Wass, S. Franks, P. Nightingale, J. W. Tomlinson, W. Arlt and P. M. Stewart (2009). "Increased 5 alpha-reductase activity and adrenocortical drive in women with polycystic ovary syndrome." J Clin Endocrinol Metab 94(9): 3558-3566. 110 How are you planning to ensure adequate supervision? Our research groups are translational groups made up of clinicians and scientists. Dr Alex Sinclair (Principal Supervisor) has a research portfolio of clinical and translational studies applied to IIH and ICP. She heads the Cerebrospinal Disorders Research Group which has developed specialised in vitro and in vivo models to investigate choroid plexus function and neuroendocrine dysregulation. This lab also has a number of translational laboratory studies running at this time. The student will work with the CSF disorders research group and will be supported by weekly supervisor meetings. Additionally, day to day support and coaching will come from the other members of the CSF research group (post doc, PhD student, senior lecturer and Clinical Research Fellow). The project will fit into the portfolio of studies (both basic science and clinical) conducted by the group. The student will benefit from state of the art equipment, excellent training and a supportive environment. The student role. The student will be part of the CSF Disorders Research Group and will contribute to discussions and present their work in the weekly lab meetings. As part of the team the student will be able to interact on a daily basis with other members of the group and will have the opportunity of learning how medical knowledge is acquired in the laboratory setting. The student will learn a variety of molecular biology, immunohistochemical, cell culture techniques. The student will also utilise clinical samples collected from IIH patient (translational work from existing ongoing clinical studies). Skills in data interpretation, statistical analysis and writing will also be acquired. If successful, the work will likely lead to a peer reviewed publication and opportunities to present the work at local and national conferences. 111 Lead Supervisor: Dr Zania Stamataki Contact Email: Telephone: z.stamataki@bham.ac.uk 01214146967 Co Supervisor: Dr Patricia Lalor Project Title: T cell entosis in the liver: the impact of inflammation. Department: Immunity and Infection Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? Potentially. Project Outline Entosis is the process whereby one cell invades another, and can remain internalised in its own vesicle in the host cell’s cytoplasm for long periods of time. The internalised cell may die or it may be released unharmed, and little is known regarding the molecules guiding these decisions. We recently discovered that T cells internalise into hepatocytes in the liver via entosis. This may have implications for the role of T cells in the regulation of liver immunity, and it is therefore a hot topic in immunology as well as cell biology. The successful candidate will learn how to coculture human hepatocyte and lymphocyte cell lines, label them with fluorescent markers and measure internalised and released cells by flow cytometry. Lymphocyte entosis will be performed in the presence of a series of proinflammatory cytokines, anti-inflammatory mediators and drugs relevant in liver inflammation to help identify the conditions of lymphocyte release from hepatocytes. The techniques used have already been established in our lab. References Overholtzer, M. and J.S. Brugge, The cell biology of cell-in-cell structures. Nat Rev Mol Cell Biol, 2008. 9(10): p. 796-809. 2. Overholtzer, M., et al., A nonapoptotic cell death process, entosis, that occurs by cell-in-cell invasion. Cell, 2007. 131(5): p. 966-79. 3. Benseler, V., et al., Hepatocyte entry leads to degradation of autoreactive CD8 T cells. Proc Natl Acad Sci U S A, 2011. 108(40): p. 1673540. 1. How are you planning to ensure adequate supervision? The successful candidate will work directly with the primary supervisor (Dr Zania Stamataki) who is an early career research scientist that spends a lot of her time in the laboratory. The student will work closely with Zania and PhD students with an interest in T cell entosis and T cell biology. The project will take part in the liver labs, a vibrant research environment where the student will have the opportunity to interact with postgraduate students (MRes, MD and PhD), postdoctoral scientists and clinical and non-clinical researchers. Weekly lab meetings will provide the opportunity to liaise with Prof. David Adams and other members of the lab to have input in the project, and will be also a valuable forum for the student to hear about the progress of other projects in our lab. The student role. 112 The successful candidate will join a highly productive research lab with expertise in basic/translational and clinical research. Key research interests in the Liver Labs involve ongoing projects in immunology, hepatology and virology so there is plenty of opportunity for the student to sample multiple research areas. During their time in the lab, the student will be trained in laboratory techniques relevant to the project and learn how to perform experiments in a quality-controlled manner. Beyond research excellence, the intellectual contribution of the student to this project will be strongly encouraged. The student will learn to evaluate research publications and interpret results, analyse experiments and form hypotheses with the aim to foster the ability to place their research into “the bigger picture” in the field. On a daily basis, the student will plan and perform experiments, analyse results and discuss their findings in brainstorming sessions with other scientists. As the project progresses, experimental data may be put together for presentation in scientific conferences and as part of scientific publications. 113 Lead Supervisor: Professor David Thickett Contact Email: Telephone: a.scott@bham.ac.uk or d.thickett@bham.ac.uk Co Supervisor: Dr. Aaron Scott Project Title: The effect of e-cigarette smoking on macrophage function in acute inflammation Department: Department of Clinical Respiratory Sciences Centre for Translational Inflammation Research Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Macrophages play a critical role in sepsis, lung injury and inflammation. They are derived from peripheral blood monocytes and the bone marrow and are recruited to sites of tissue injury and infection forming the first line of defense towards pathogen and particulate exposure, acting to induce and perpetuate innate and adaptive immune responses [1] E-cigarettes (ECIG) have recently become popular as a substitute for and as a tool in smoking cessation. As these systems deliver nicotine without combusting tobacco these vapour devices undoubtedly reduce the exposure to carcinogens from other agents in cigarette smoke. However the negative impact of these devices remains to be fully explored. Murine studies examining asthmatic airway inflammation (AI) and airway hyperresponsiveness (AHR) have shown e-cigarette (ECIG) cartridge fluid can increase inflammatory cell infiltration into the airways and increased inflammatory cytokine production [2] Recent work examining gene expression in human bronchial epithelial cells has shown similarities in gene expression when cells are exposed to ECIG fluid when compared to cigarette smoke-conditioned media[3]. In agreement with the murine studies, high levels of nicotine from ECIG-conditioned media has also been shown to induce a proliferative, invasive phenotype in human bronchial epithelial cells in an air-liquid interface model [3]. Many practitioners actively recommend these nicotine delivery systems, especially to those at risk of developing respiratory problems and yet relatively little is known about the effect on normal biology and importantly the effects in acute inflammation and resolution. This project therefore aims to investigate the effects of smoking and ECIG fluid on the function of monocytes and macrophages from both healthy volunteers, patients undergoing lung resection surgery and patients suffering from ALI/ARDS. HYPOTHESIS Smoking and ECIGs supress macrophage function and therefore cause increase in post-operative complications and poor outcomes from ARDS. AIMS: 1] To investigate the effect of ECIG-fluid on phagocytosis and inflammatory 114 response to stimulation in monocyte derived macrophages (from healthy volunteers) and primary human alveolar macrophages 2] To investigate the mechanism of action of smoking versus non-smoking on macrophages from patients undergoing lung resection surgery and in those with ARDS to determine if this correlates to surgical complication rates and ARDS outcomes. References 1. Herold S, Mayer K, Lohmeyer J: Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol 2011, 2:65. 2. Lim HB, Kim SH: Inhallation of e-Cigarette Cartridge Solution Aggravates Allergen-induced Airway Inflammation and Hyperresponsiveness in Mice. Toxicol Res. 2014 Mar;30(1):13-8. 3. Stacy J. Park, Tonya C. Walser, Catalina Perdomo, Teresa Wang, Paul C. Pagano, Elvira L. Liclican, Kostyantyn Krysan, Jill E. Larsen, John D. Minna, Marc E. Lenburg, Avrum Spira, Steven M. Dubinett. The effect of ecigarette exposure on airway epithelial cell gene expression and transformation. [abstract]. In: Proceedings of the AACR-IASLC Joint Conference on Molecular Origins of Lung Cancer; 2014 Jan 6-9; Clin Cancer Res 2014;20. How are you planning to ensure adequate supervision? Both the supervisors are located in the new Centre for Translational Inflammation Research in the University Labs in the new QEHB. Weekly supervisor meetings will be arranged to ensure student progress and to plan the evolution of the project. The student will attend respiratory research in progress meetings weekly. Considerable expertise exists within the respiratory research group in the techniques necessary to make this project successful and have extensive experience of BMedSci student supervision with a good track record with 1st class degree results as well as outputs with publications and abstracts presented at national and international meetings. Our most recent student has not only won the best BMedSci project award but also a British Thoracic Society Medical Student Prize and is an ambassador award to promote the Clinical Intercalated BMedSci degree. Patient samples are available for the primary alveolar macrophage work in collaboration with Mr Babu Naidu, thoracic surgeon and Sepsis/ARDS blood samples from other trials ongoing within the group and in collaboration with Dr David Thickett and Dr Elizabeth Sapey. Regular updates of progress with these collaborators will ensure detailed multidisciplinary support for the successful student. The student role. The student will learn the cell isolation and culture techniques to obtain monocyte derived macrophages from healthy and sepsis/ARDS blood, and primary alveolar macrophages from lung resection tissue samples and bronchoalveolar lavage fluid. The student will perform stimulation experiments and perform phagocytosis assays and measure markers of inflammation by ELISA and phenotype the macrophages by flow cytometry. 115 Lead Supervisor: Dr. Kai-Michael Toellner Contact Email: Telephone: Email: k.m.toellner@bham.ac.uk Telephone: +44 (0)121 415 8687 Co Supervisor: Laura Garcia Ibanez Project Title: Migration Events from the Germinal Centre during Vaccine Responses Department: School of Immunity and Infection Will the project require a Home Office working with animals licence? No, can be acquired Is the Project Cancer related? No Project Outline Vaccination results in the production of high affinity protective antibody. The differentiation of high affinity memory B cells and plasma cells occurs in germinal centres (GC) in secondary lymphoid organs. Affinity maturation involves migration between the GC’s dark and light zones, and also migration of effector cells out of the GC. The signals that indicate to a B cell that it is time to differentiate and leave the GC are far from being fully understood. Chemokines, small secreted proteins that regulate leukocyte migration, are prime candidates to regulate this exit. It has been shown that chemokines have key roles in B cell differentiation and migration. We will evaluate the effect of the deficiency of chemokine receptor CCR7 on the exit of plasma cells and memory B cells from the spleen to other organs such as lymph nodes, blood and bone marrow. CCR7 binds to chemokines CCL19 and CCL21, produced by stromal cells in the vicinity of GCs. Mouse models deficient for CCR7 on B cells are available. Preliminary data shows that CCR7-deficient antigen-specific B cells form different memory B cell populations than CCR7-sufficient antigen-specific B cells but the relevance of these changes in memory B cell populations is unclear. The response of these memory B cells to subsequent antigen encounters may be affected and needs to be studied. The project will help to understand migration and differentiation of effector cells, especially of the different subsets of memory B cells, which may lead to new ways of manipulating antibody producing cells in vaccine responses. References Allen, C. D., T. Okada, J. G. Cyster (2007). “Germinal-center organization and cellular dynamics.” Immunity 27(2): 190-202. Comerford, I., Y. Harata-Lee, M. D. Bunting, C. Gregor, E. E. Kara, S. R. McColl (2013). “A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptative immune system.” Cytokine Grow Factor Rev 24(3): 269-283. Zhang, Y., M. Meyer-Hermann, L. A. George,M.T. Figge, M. Khan, M. Goodall, S. P. Young, A. Reynolds, F. Falciani, A. Waisman, C. A. Notley, M.R. Ehrenstein, M. Kosko-Vilbois, K. M.Toellner (2013). “Germinal center B cells govern their own fate 116 via antibody feedback.”J Exp Med 11;201(3): 457-464 How are you planning to ensure adequate supervision? All the experiments will be planned in regular meetings between student, PhD student co-supervising and main supervisor ensuring the logical progress of the project. Experiments performed in the lab will be directly supervised and guided by PhD student, who is expert in all techniques and procedures involved, and works on a closely related project with similar questions covering other chemokine receptors. Weekly meetings will be set up to ensure the satisfactory progress of the project and to deal with any difficulties that may have arisen. Further, our research group has weekly lab meetings, where problems are put in common with all the members of the lab and weekly journal clubs, where the most recent and relevant papers in the field are discussed. The student role. The student will receive full training in the following techniques: murine tissue preparation (spleen, lymph nodes, bone marrow and blood), flow cytometry, immunohistochemistry, light and fluorescence microscopy, ELISA and data analysis. The student will be directly involved in lab meetings, having the opportunity to discuss their work with the rest of the group for feedback and possible new ideas, in order to train in scientific discussion and project planning. Also, he will participate in journal clubs, giving him the possibility to improve presentation skills in a dissented environment. Procedures on animals can be carried out by PhD student, and a Home Office license to perform procedures on animals can be acquired during the course of the project. 117 Lead Supervisor: Dr Zania Stamataki Contact Email: Telephone: z.stamataki@bham.ac.uk 01214146967 Co Supervisor: Dr Gary Reynolds Project Title: Deciphering the B cell compartment in human liver disease. Department: Immunity and Infection Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? No Project Outline The liver is the largest internal organ in the human body and performs ~500 important functions. Blood traffics continuously through this organ, yet little is known about its contribution to immunity. B cells are important lymphocytes as they perform essential immune functions that protect against pathogens via i) antibody production, ii) antigen presentation to T cells, iii) cytokine expression, and others. This project is designed to investigate the distribution of B cell subsets in human explanted livers from patients with end stage liver disease. Outcomes from this work will help identify B cell differences between chronic liver diseases of viral origin, autoimmune aetiology or other forms of liver injury. We are based at the Institute for Biomedical Research, adjacent to the New Queen Elisabeth Hospital and the University of Birmingham Medical School. Our group benefits from access to well-characterised patient cohorts that allow unique insights into liver disease. The successful candidate will be part of a multidisciplinary environment of biologists, immunologists, virologists and clinicians that work together to extend our knowledge about liver immunity, in health and in inflammation. References Microanatomy of the liver immune system. Nemeth E, Baird AW, O'Farrelly C. Semin Immunopathol. 2009 Sep;31(3):333-43. Human B cell subsets. Jackson SM, Wilson PC, James JA, Capra JD. Adv Immunol. 2008;98:151-224. How are you planning to ensure adequate supervision? The successful candidate will work directly with the primary supervisor (Dr Zania Stamataki) who is an early career research scientist that spends a lot of her time in the laboratory. The student will work closely with Sudha Purswani, a final year PhD student that has an interest in B cell biology. The project will take part in the liver labs, a vibrant research environment where the student will have the opportunity to interact with postgraduate students (MRes, MD and PhD), postdoctoral scientists and clinical and non-clinical researchers. Weekly lab meetings will provide the opportunity to liaise with Prof. David Adams and other members of the lab to have input in the project, and will be also a valuable forum for the student to hear about the progress 118 of other projects in our lab. The student role. The successful candidate will join a highly productive research lab with expertise in basic/translational and clinical research. Key research interests in the Liver Labs involve ongoing projects in immunology, hepatology and virology so there is plenty of opportunity for the student to sample multiple research areas. During their time in the lab, the student will be trained in laboratory techniques relevant to the project and learn how to perform experiments in a quality-controlled manner. Beyond research excellence, the intellectual contribution of the student to this project will be strongly encouraged. The student will learn to evaluate research publications and interpret results, analyse experiments and form hypotheses with the aim to foster the ability to place their research into “the bigger picture” in the field. On a daily basis, the student will plan and perform experiments, analyse results and discuss their findings in brainstorming sessions with other scientists. As the project progresses, experimental data may be put together for presentation in scientific conferences and as part of scientific publications. 119 Lead Supervisor: Richard Tuxworth Contact Email: Telephone: r.i.tuxworth@bham.ac.uk 40476 Co Supervisor: Tim Barrett Project Title: Lysosomal storage disorders: genetic models of neurodegeneration. Department: Clinical and Experimental Medicine Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline Lysosomes are low pH organelles central to the regulation of growth, autophagy and catabolism. Dysfunction of lysosomes is thought to contribute to most or all neurodegenerative diseases but its relative importance is difficult to ascertain in the common late-onset neurodegenerative disorders which are complex and multifactorial. The lysosomal storage disorders (LSDs) are a group of approximately 50 inherited diseases that result in lysosomal failure in cells. Early-onset neuronal pathology occurs in many of the LSDs, including neurodegeneration. Since each disease is caused by a single gene defect, similar mutations can be generated in genetically tractable animals such as mice and fruit flies to use as simple models to study the cell biology and synaptic biology of neurodegeneration. This project will use a combination of fruit fly genetics, cell culture, biochemistry and cell biology to ask what is special about the lysosomes of neurons that makes them particularly vulnerable in the LSDs. References Kollmann K, Uusi-Rauva K, Scifo E, Tyynelä J, Jalanko A, Braulke T. 2013 Cell biology and function of neuronal ceroid lipofuscinosis-related proteins. http://www.ncbi.nlm.nih.gov/pubmed/23402926 Hindle S, Hebbar S, Sweeney ST. 2011 Invertebrate models of lysosomal storage disease: what have we learned so far? http://www.ncbi.nlm.nih.gov/pubmed/22038288 How are you planning to ensure adequate supervision? Richard Tuxworth will supervise full-time. The student role. The student will generate novels mutations, markers and cell biological probes in cells and flies to examine how complexes of lysosomal membrane proteins differ as the environment changes. The student will learn a broad range of molecular, cell biological and genetic techniques. 120 Lead Supervisor: Dr Graham Wallace Contact Email: Telephone: g.r.wallace@bham.ac.uk 0121 371 3255 Co Supervisor: Miss Saaeha Rauz Project Title: Does endogenous cortisol induce miRNA Department: Ophthalmology, Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No Project Outline The eye is regarded as an immune privileged site, due to effective barriers and an immunosupprssive microenvironment. We have shown that ocular barrier cells can convert inactive cortisone to active cortisol. Related to this we have previously shown that treatment of human corneal fibroblasts with dexamethsone, a synthetic glucocoticoid, induced the expression of several miRNA. These are small RNA molecules that bind to mRNA to control translation. Among the miRNA identified included two associated with fibrosis. This is of interest as topical treatment with dexamethasone can caused raised intraocular pressure that may be associated with fibrosis. In this project we wish to determine whether endogenous cortisol production mediates the same changes in miRNA expression. Primary corneal cells will be prepared from tissue rims, following transplant. Cells will be treated with cortisone and miRNA expression will be assessed by a fluorometric assay. Target mRNAs will be identified by analysing on-line databases and candidate genes expression will be assessed by PCR. The potential for stress responses to alterprotein expression in corenal cells is intriguing and potentially important in controlling site-threatening inflammatory disease. References Susarla R, Liu L, Walker EA, Bujalska IJ, Alsalem J, Williams GP, Sreekantam S, Taylor AE, Tallouzi M, Southworth HS, Murray PI, Wallace GR, Rauz S. Cortisol biosynthesis in the human ocular surface innate immune response. PLoS One. 2014 Apr 15;9(4):e94913 Liu L, Walker EA, Kissane S, Khan I, Murray PI, Rauz S, Wallace GR. Gene expression and miR profiles of human corneal fibroblasts in response to dexamethasone. Invest Ophthalmol Vis Sci. 2011 Sep 21;52(10):7282How are you planning to ensure adequate supervision? The student will be supervised on a daily basis by Dr Wallace in the Centre for Translational Inflammation laboratories in the QE Hospital. Weekly meeting with both supervisors will take place every Wednesday morning. Miss Rauz will supervise the tissue collection at the Birmingham and Midland Eye centre, City Hospital. The student role. 121 The student will prepare the corneal cells from tissue rims and grow under tissues culture conditions. Test for miRNA using a commercial fluorometric kit. Analyse miRNA databases for potential targets PCR and protein analysis (ELIsA) of potential targets 122 Lead Supervisor: Dr Doug Ward Contact Email: Telephone: d.g.ward@bham.ac.uk 01214149528 Co Supervisor: Dr Rik Bryan and Prof Jean-Baptiste Cazier Project Title: Correlating the genomic landscape of bladder cancer with clinical outcome. Department: School of Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Bladder cancer is the 5th most common cancer in Western societies, responsible for 10,000 new cases and 5,000 deaths annually in the UK. Patient management and treatment have not changed significantly for decades and the prognosis is dismal for patients with muscle-invasive disease. 75% of new cases of bladder cancer are non-muscle invasive and the majority of these are papillary tumours with activating mutations in the FGFR3–PIK3CA pathway. Most cases of muscle invasive disease carry mutations inactivating tumour suppressors. Within both invasive and non-invasive disease there is considerable heterogeneity in gene mutation, gene expression and clinical behaviour. Next generation sequencing data is now available for several hundred bladder tumours painting a complex picture with more than 30 genes mutated at statistically significant frequency in these patients and on average several hundred genes are mutated in each tumour. We have collected tumour tissue and matched whole blood from 1000 patients with bladder cancer and well collated outcome data. We are generating exome and RNASeq data on a large number of these tumours with a focus on particularly difficult to manage clinical sub-groups. The aim of this project is to analyse these datasets in the context of outcome to develop prognostic and predictive biomarker signatures. References Whole-genome sequencing of bladder cancers reveals somatic CDKN1A mutations and clinicopathological associations with mutation burden. Cazier JB, Rao SR, McLean CM, Walker AL, Wright BJ, Jaeger EE, Kartsonaki C, Marsden L, Yau C, Camps C, Kaisaki P; Oxford-Illumina WGS500 Consortium, Taylor J, Catto JW, Tomlinson IP, Kiltie AE, Hamdy FC. Nat Commun. 2014 5 3756 Combined proteome and transcriptome analyses for the discovery of urinary biomarkers for urothelial carcinoma. Shimwell NJ, Bryan RT, Wei W, James ND, Cheng KK, Zeegers MP, Johnson PJ, Martin A, Ward DG. Br J Cancer. 2013 108 1854-61. How are you planning to ensure adequate supervision? Daily office/laboratory supervision and fortnightly meetings with all supervisors. 123 The student role. The student will perform bioinformatic analyses of next generation sequencing data to aid in our understanding of how gene copy numbers, mutations and expression levels influence phenotype in bladder cancer. This data will be used to develop clinically relevant predictive models to aid in the development of precision medicine in bladder cancer. If the student wishes the project may also contain a wet-lab element using targeted re-sequencing, qPCR, Western blotting and possibly mass spectrometry to confirm and extend the NGS data. 124 Lead Supervisor: Professor Hisham Mehanna Contact Email: Telephone: Co Supervisor: h.mehanna@bham.ac.uk 0121 414 6547 (Mrs Gemma Jones, PA to Hisham Mehanna) Prof Jean-Baptiste Cazier Project Title: In Silico methylation analysis of head and neck Department: Cancer Sciences cancer. Will the project require a Home Office working with animals licence? NO Is the Project Cancer related? Yes Project Outline The Institute of Head and Neck Studies and Education (InHANSE), led by Professor Hisham Mehanna, focuses on research into diseases of the head, neck and thyroid, and on the education of health professionals in the field. For head and neck cancer one of the most complex areas of management is the choice of primary treatment (surgery vs chemo-therapy) and this often relies on clinical factors (mainly clinical stage) and the therapeutic preference of the treating centre. InHANSE are near completion of a clinical trial Predictr, this is a large multicentred trial examining the differential expression of 10 biomarkers between normal, pre-malignant and malignant tissue. The aim of this trial is to utilise the biological characteristics of a tumour alongside clinical factors to more reliably predict those cases that have a high risk of transformation. This will allow more aggressive treatment of high risk cases, whilst sparing the majority of patients from potentially disabling side effects. Epigenetic modification of DNA has been increasingly recognized as performing an important role in carcinogenesis. However, there are suprisingly few studies of genome-wide methylation and its effects in head and neck cancer. In order to improve the prognostic classifier obtained from the Predictr trial we are currently investigating methylation status in head and neck cancers compared to normal tissue. In collaboration with the National Cancer Institute of Brazil (INCA) we have preliminary data showing differential methylation between normal, pre-malignant and malignant tissue as well as HPV- and HPV+ disease. The aims of this project are two fold: 1. Validate our in vitro methylation findings using in silico database searches 2. Use in silico database searches to find novel methylation gene signatures that will predict tumours that are high risk. References Mehanna H et al. Head and neck cancer--Part 2: Treatment and prognostic factors. Bmj. 2010;341:c4690. Nejat Dalay. Role of DNA methylation in head and neck cancer. Clin Epigenet (2011) 2:123-150. Beggs A et al. Whole-genome methylation of benign and malignant colorectal tumours. J Pathol (2013)229:697-704. 125 Cazier, J.B. et al. Whole-genome sequencing of bladder cancers reveals somatic CDKN1A mutations and clinicopathological associations with mutation burden. Nat Commun (2014) 5, 3756. How are you planning to ensure adequate supervision? InHANSE is a team of over 15 researchers, which will include a bioinformatician by January 2015. The student will be primarily supervised within InHANSE but will also co-supervised by Professor Jean-Baptiste Cazier, the Director Designate of the Centre for Computational Biology, with the involvement of Dr Andrew Beggs who runs a bioinformatics “dry-lab” to search for novel therapeutic targets and stratification markers in colorectal cancer. The student will have 1 to 1 meetings 2-weekly with the InHANSE bioinformatician and monthly meetings with Professor Mehanna and/or Professor Cazier. The student role. The student will learn to use and interrogate publicly available, online genomics and methylation data repositories bases, and carry out analyses and meta-analysis on the data, examining hypotheses developed from our pilot data. They will examine potential associations and risk factors, as well undertake survival analyses. They will liaise and collaborate with our colleagues in INCA Brazil to analyse the extension of the pilot data. 126 Lead Supervisor: Professor Hisham Mehanna Contact Email: Telephone: h.mehanna@bham.ac.uk 0121 414 6547 (Mrs Gemma Jones, PA to Hisham Mehanna) Dr Rachel Watkins Co Supervisor: Project Title: Accelerated discovery and development of novel therapeutic agents for the treatment of head and neck cancer patients Department: Cancer Sciences Will the project require a Home Office working with animals licence? Yes Is the Project Cancer related? Yes Project Outline In the last 30 years, there have been only 2 new anti-cancer drugs introduced for the treatment of head and neck cancer. As a result, traditionally the survival of patients with advanced head and neck cancer has been very poor, with only a 50% survival rate. These survival rates have not increased significantly in the past 30 years. A key goal of Accelerated is to increase the speed with which these new treatments are implemented into clinical practice, whilst reducing the costs associated with identification, testing and development. The Accelerated project provides a platform for the identification and validation of novel drugs for the management of head and neck cancer through examining currently approved, non-cancer, off-patent drugs for anti-cancer activity. Potential therapeutic agents are taken through a series of in vitro cell-based assays to determine their efficacy against head and neck cancer survival and proliferation. These studies are carried out using a panel of cell lines and primary cultures derived from donated patient tissues, and include proliferation assays, migration assays, flow cytometry and 3D organotypic cultures. Following on from this in vivo xenograft mouse models will be used to confirm the efficacy of the candidate agents in living organisms. Once targets have been identified and confirmed we will explore their mechanism of action using a range of molecular biology techniques. References Mehanna H et al. Head and neck cancer--Part 2: Treatment and prognostic factors. Bmj. 2010;341:c4690 Fung C et al. Emerging drugs to treat squamous cell carcinomas of the head and neck. Expert opinion on emerging drugs. 2010;15(3):355-73 DiMasi JA et al. The price of innovation: new estimates of drug development costs. Journal of health economics. 2003;22(2):151-85 127 Bodmer M et al. Long-term metformin use is associated with decreased risk of breast cancer. Diabetes care. 2010;33(6):1304-8. Wu Z et al. Quantitative chemical proteomics reveals new potential drug targets in head and neck cancer. Molecular & cellular proteomics: MCP. 2011;10(12):M111 011635 How are you planning to ensure adequate supervision? The Accelerated platform was established in 2013. Currently there are 3 postdocs and 2 research technicians working full time on this project, in total there will be 5 members of staff to help with Supervision. The translation team have informal weekly meeting’s to discuss data and to plan the following weeks experiments. These meeting’s are supported by fortnightly meetings, in which data is presented and discussed in detail. In addition the student has 1 to 1 meetings 2-weekly meetings with Dr Watkins the team leader and monthly meetings with Prof Mehanna The student role. The student will participate in the delivery of the Accelerated platform. Using a panel of characterised head and neck cancer cell lines they will screen agents for efficacy against HNC survival and proliferation using high throughput Alamar blue proliferation assays. They will test agents alone and in combination with chemotherapy and radiotherapy to mimic current treatment in head and neck. They will then validate any hits further with a series of in vitro cell based assays such as clonogenic, scratch-wound migration, apoptosis and cell cycle analysis. Those agents that show promise in the in vitro models will then be tested in head and neck xenograft animal models to verify their efficacy against head and neck cancer tumours in living organisms. 128 Lead Supervisor: Prof Ben Willcox (http://ciic.org.uk/team-member/prof-ben-willcox/) Contact Email: Telephone: 0121 414 9533/07919167723 Co Supervisor: Dr Carrie Willcox, Dr Fiyaz Mohammed Project Title: Exploring recognition and manipulation of stressinduced ligands by the NKG2D immunoreceptor. Department: School of Cancer Sciences Will the project require a Home Office working with animals licence? No Is the Project Cancer related? YES Project Outline The project focusses on NKG2D, a key activatory receptor expressed on immune effector cells, that recognises a family of related MHC-like ligands that are upregulated after cellular stress, such as infection or tumourigenesis. Ligand recognition by NKG2D is important in cancer immunosurveillance, and might be exploitable for cancer immunotherapy, however it is currently poorly understood. This project builds on research by Prof Willcox’ and Prof Moss’ research groups, and aims to: (i) Assess which NKG2D ligands (NKG2DL) are the most potent activators of immune effector cells. (ii) Structure-based approach to defining a core recognition motif on NKG2DL that is conserved across the family. (iii) High-throughput screening approach to identify how to therapeutically manipulate expression of the most bioligically potent NKG2DL. References Zuo-J, Willcox-CR, Mohammed-FM, Antoun-A, Malladi-R, Inman-C, Croudace-J, Briggs-D, Willcox-BE, Moss-PA ULBP6 polymorphism modulates NKG2D-mediated immunity by creating an ultrastable NKG2D receptor/ligand interaction Nature Immunology in preparation How are you planning to ensure adequate supervision? There are four excellent postdoctoral scientists who will play a role in supervising the student. Dr Jianmin Zuo is an excellent cellular immunologist focusing on the role of NKG2D in transplantation; Dr Carrie Willcox is an expert on cellular stress recognition with outstanding molecular and cellular skills; Dr Martin Davey will assist Dr Zuo in cellular assays. Dr Fiyaz Mohammed is a structural biologist with a strong interest in immune receptor/ligand interactions. The student role. The student will gain core laboratory skills in a wide range of techniques, including molecular skills in construct design and generation, molecular modelling, generation of transfectants, and cellular activation assays; also, time permitting, in high throughput screening approaches to identify chemotherapy drugs that upregulate key NKG2D ligands. 129 Lead Supervisor: Jayne Wilson Co Supervisor: Professor Keith Wheatley, Professor Pam Kearns Project Title: Systematic reviews of treatment strategies for hepatoblastoma in children and young adults Department: CRCTU Contact Email: Telephone: J.S.Wilson.1@bham.ac.uk 0121 414 3461 Will the project require a Home Office working with animals licence? No Is the project cancer related? Yes Discipline: Cancer Studies Histopathology Endocrinology Haematology Infection Immunology & Renal Rheumatology / Orth Evolutionary Med Liver & GI Medicine Project Outline Hepatoblastoma is a very rare childhood cancer of unknown aetiology. Approximately 8 children a year are diagnosed in the UK. The main treatment is surgery but chemotherapy is used to shrink the tumour prior to surgery or treat unresectable or metastatic disease. Survival estimates are very good for patients with local disease but decrease significantly in patients with advanced or metastatic disease. The aim of this project is to identify trials and studies that have reported the efficacy and effectiveness of therapeutic interventions for hepatoblastoma. We aim to focus on chemotherapy and will look at single arm studies as well as controlled trials. These systematic reviews will help inform clinical practice and contribute to defining the pertinent clinical research questions to be addressed in future trials in order to improve the treatment for these diseases. References References on why you need a systematic review in clinical trials Clarke M, Hopewell S, Chalmers I. Clinical trials should begin and end with systematic reviews of relevant evidence: 12 years and waiting. Lancet 2010; 376(9734):20-21. Goudie AC, Sutton AJ, Jones DR, Donald A. Empirical assessment suggests that existing evidence could be used more fully in designing randomized controlled trials. J Clin Epidemiol 2010; 63(9):983-991. Guides to undertaking a systematic review. Systematic Reviews – CRD’s guidance for undertaking reviews in health care. Centre for Reviews and Dissemination. University 130 of York 2009 ISBN: 978 1 900640 47 3 http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf pragmatic guide to how to do SR Cochrane Handbook for Systematic Reviews of Interventions. Julian PT Higgins, Sally Green. Wiley Blackwell 2008 ISBN: 978 0 470 69951 5 http://www.cochrane.org/training/cochrane-handbook mainly deals with RCTs but does have info on other designs Hepatoblastoma Herzog CE, Adnrassy RJ, Eftekari F., Childhood Cancers: Hepatoblastoma. The Oncologist 2000 5:445-453. Pinkerton R, Shankar A, Matthay KK., Evidence-based pediatric oncology. Third Edition. Wiley-Blackwell. 2013 How are you planning to ensure adequate supervision? Keith Wheatley is Professor of Medical Statistics at CRCTU and statistical lead for the Children’s Cancer Trials Team (CCTT) and Jayne Wilson is a Senior Systematic Reviewer at CRCT. Both have substantial experience and expertise in evidence synthesis that will enable us to supervise and educate the student(s) appropriately. Supervision will be in the form of regular meetings. The student will also have the opportunity to attend training courses in systematic review methods. The student role. The student will learn through working on their systematic review and participation in meetings to discuss the review. The student will learn how to design and conduct a systematic review. The student will Write the review protocol, learning about how to formulate an answerable question. Systematically search for studies using electronic databases and reference manager software. Data extract information and critically appraise the studies included in the review. By the end of the review the student will learn about the different statistics used in research papers and how to interpret them, they will also learn about different trial designs and how to recognise whether a study has been well conducted. Synthesize the data using statistical techniques such as meta-analysis. Write up findings in a scientific format, but also learn who to make findings accessible to users of systematic reviews including patients and clinicians. The student will also learn about how systematic reviews are used in clinical decision making process’s, learn about the institutions who produce and use systematic reviews such as NICE and the Cochrane Collaboration and learn how systematic reviews are used in the development of clinical trials. 131 Lead Supervisor: Jayne Wilson Co Supervisor: Dr Neil Steven, Mr Neil Sharma, Miss Lisha McClelland Project Title: Systematic review of management of sinonasal melanoma Department: CRCTU Contact Email: Telephone: J.S.Wilson.1@bham.ac.uk 0121 414 3461 Will the project require a Home Office working with animals licence? No Is the project cancer related? Yes Discipline: Cancer Studies Histopathology Endocrinology Haematology Infection Immunology & Renal Rheumatology / Orth Evolutionary Med Liver & GI Medicine Project Outline Sinonasal melanoma is a rare malignancy with a poor prognosis, and its aggressive nature is highlighted by the omission of stage I and II mucosal melanomas from the UICC/AJCC TNM Classification of Malignant Tumours. Primary management is surgical, and although the previously held view that these cancers were radioresistant has changed, there is no clear consensus regarding use of radiation or chemotherapy. Adjuvant therapy has not led to increased prognosis, with high rates of loco-regional recurrence and metastases and low 5 year survival. The aim of this project is to work with a cross disciplinary team to develop clinical guidelines for managing sino-nasal melanoma and explore the feasibility of clinical trials investigating potential novel therapeutic strategies. The objectives are: A. to undertake a systematic review of publications reporting surgical, radiation, cytotoxic, immune and molecularly targeted therapeutic interventions for sinonasal melanoma. B. to carry out a review of the presentation, investigation (including molecular phenotyping) and management of the sino-nasal melanoma at University Hospital Birmingham. References References on why you need a systematic review in clinical trials Clarke M, Hopewell S, Chalmers I. Clinical trials should begin and end with systematic reviews of relevant evidence: 12 years and waiting. Lancet 2010; 376(9734):20-21. Goudie AC, Sutton AJ, Jones DR, Donald A. Empirical assessment suggests that existing evidence could be used more fully in designing randomized controlled trials. 132 J Clin Epidemiol 2010; 63(9):983-991. Guides to undertaking a systematic review. Systematic Reviews – CRD’s guidance for undertaking reviews in health care. Centre for Reviews and Dissemination. University of York 2009 ISBN: 978 1 900640 47 3 http://www.york.ac.uk/inst/crd/pdf/Systematic_Reviews.pdf pragmatic guide to how to do SR Cochrane Handbook for Systematic Reviews of Interventions. Julian PT Higgins, Sally Green. Wiley Blackwell 2008 ISBN: 978 0 470 69951 5 http://www.cochrane.org/training/cochrane-handbook mainly deals with RCTs but does have info on other designs Sinonasal melanoma Gore MR, Zanation AM, Survival in Sinonasal Melanoma: A Meta-analysis. J Neurol Surg B Skull Base. 2012 Jun;73(3):157-62 Roland NJ, Paleri V (eds). Head and Neck Cancer: Multidisciplinary Management Guidelines. 4th edition. London: ENT UK; 2011 How are you planning to ensure adequate supervision? This project is led by a multidisciplinary panel of supervisors who will ensure a well rounded approach to the project with supervision in the form of regular meetings and electronic updates. The student will also have the opportunity for formal and practical training in systematic review methods. Clinically, the student will attend diverse clinics including rhinology and oncology and join the multidisciplinary team discussing case management. The student will meet and speak to patients suffering from sinonasal melanoma, giving them a unique insight into the patient perspective. The supervisory team comprise: Jayne Wilson is a Senior Systematic Reviewer at CRCT with substantial experience and expertise in evidence synthesis that will ensure the student is supervised and educated appropriately. Neil Steven is a Consultant Oncologist and Deputy Clinical Director of the CRUK Clinical Trials Unit with considerable experience in the use of cytotoxic, molecular targeted and immune therapies for melanoma. Neil Sharma is an NIHR Clinical Lecturer in ENT at the Institute of Head and Neck Studies and Education, involved in clinical trials relating to head and neck cancers. Lisha McClelland is a Consultant ENT/Sinonasal surgeon frequently treating patients with this disease. The student role. 133 The student will learn through working on their systematic review and participation in meetings to discuss the review. The student will learn how to design and conduct a systematic review. The student will Write the review protocol, learning about how to formulate an answerable question. Systematically search for studies using electronic databases and reference manager software. Data extract information and critically appraise the studies included in the review. By the end of the review the student will learn about the different statistics used in research papers and how to interpret them, they will also learn about different trial designs and how to recognise whether a study has been well conducted. Synthesize the data using statistical techniques such as meta-analysis. Write up findings in a scientific format, but also learn who to make findings accessible to users of systematic reviews including patients and clinicians. The student will also learn about how systematic reviews are used in clinical decision making process’s, learn about the institutions who produce and use systematic reviews such as NICE and the Cochrane Collaboration and learn how systematic reviews are used in the development of clinical trials. The student will also learn how to extract and systematise diverse clinical, radiological and pathological data from case records. 134 Lead Supervisor: Dr Jonathan W Mueller (CEDAM) Co Supervisor: Dr Daniel A Tennant (Cancer Sciences) Prof Wiebke Arlt (CEDAM) Hypoxia as a regulator of steroid synthesis and sulfation Project Title: Department: Contact Email: Telephone: CEM – Clinical and Experimental Medicine CEDAM – Centre for Endocrinology, Diabetes and Metabolism j.w.mueller@bham.ac.uk x58819 Is the project cancer related? Discipline: No Cancer Studies Histopathology Endocrinology Haematology Infection X Immunology & Renal Rheumatology / Orth Evolutionary Med Liver & GI Medicine Project Outline During fetal development, the fetal zone of the adrenal gland produces large amounts of DHEAS, the sulfate ester of the crucial androgen precursor dehydroepiandrosterone (DHEA). DHEA sulfation in the adrenal requires two enzymes, PAPS synthase 2 (PAPSS2), to produce the sulfate donor PAPS, and DHEA sulfotransferase (SULT2A1, to attach the sulfate group to the DHEA molecule [1]). It is one of the mysteries in endocrinology how this fetal zone of the adrenal quickly changes after birth, significantly shrinking in size with a steep decline in circulating DHEAS levels (Fig.1A). These dramatic physiologic changes have previously been suggested to involve enhanced apoptosis [2], but the underlying mechanisms remain unclear. A previous study has provided evidence that FZ involution occurs irrespective of gestational age at birth (Fig.1.B+C), i.e. that parturition itself appears to be the most important trigger for the initiation of rapid FZ involution [3]. Figure 1: A, Schematic representation of serum levels of the major adrenal androgen dehydroepiandrosterone-sulfate (DHEA-S) over the human lifetime. Normal DHEA-S levels in adults are as high as up to 10 µM. B and C, adrenal volume decrease and drop in DHEA-S serum levels at birth coincide irrespective of gestational age. B, mean ultrasonographic adrenal volume in cubic centimeters for 26– 135 28 wk gestation () and 29–32 wk gestation (). C, mean DHEA-S serum levels (micromoles per liter) for 26–28 wk (), 29–32 wk (), and 33–35 wk gestation (). Shaded area, Normal adult reference values. Figure A adapted from Ref. 7, Figures B and C from Ref. 3. During fetal development, the adrenal is exposed to an oxygen tension of 3% (~22 mmHg). However, upon the onset of pulmonary function at birth, a significant increase in adrenal oxygenation is observed, reaching around 12% (90 mmHg (Fig.2). The human adrenal is an organ with very high vascularisation. Hence, one can approximate its tissue oxygen tension to that of capillary arterioles. The influence of the massive increase in oxygenation after birth on adrenal development has never been investigated before. This is especially noteworthy, as adrenal steroidogenesis strongly relies on oxygen [4]. Therefore in this PhD project we propose the influence of hypoxia and changes in oxygen tension on adrenal steroid synthesis and sulfation. Figure 2: Oxygen concentration in the fetal and adult adrenal (adapted from Ref. 8). Hypothesis We hypothesise that changes in oxygen tension represent a hitherto unknown modulator of steroid synthesis and sulfation and underlies the early neonatal involution of the fetal zone of the human adrenal cortex. References (supervisors) 1) Mueller JW, Shafqat N. FEBS J. 2013 Jul;280(13):3050-7. 2) Spencer SJ, Mesiano S, Lee JY, Jaffe RB. J Clin Endocrinol Metab. 1999 Mar;84(3):1110-5. 3) Ben-David S, Zuckerman-Levin N, Epelman M, Shen-Orr Z, Levin M, Sujov P, Hochberg Z. J Clin Endocrinol Metab. 2007 Jan;92(1):93-7. 4) Prasad R, Kowalczyk JC, Meimaridou E, Storr HL, Metherell LA. J Endocrinol. 2014 Jun;221(3):R63-73. 5) Wang T, Rainey WE. Mol Cell Endocrinol. 2012 Mar 31;351(1):58-65. 6) Junqueira LC, Carneiro J (Eds) Basic Histology 2002. 7) Miller and Auchus. Endocrine Rev, 2011, 32(1):81-151. 8) Wenger RH, AINS 2004, 39, S38-43. 136 How are you planning to ensure adequate supervision? The student will receive daily supervision by Dr Mueller, during the initial phase working alongside him at the bench, including a thorough introduction to cell culture techniques, transfection, fluorescence microscopy, SDS-PAGE, quantitative western blotting, nucleic acid techniques and other basic methodology as well as steroid analytics. The student will receive special training for cell culture work using the hypoxia workstations in Dr Tennant’s lab. Previous lab experience is not a prerequisite for this project, but scientific curiosity and enthusiasm for research are very much welcome. As the lab skills of the student develop, he or she will soon be able to carry out defined elements of the research project autonomously with daily feedback. In addition, the student will participate and present at the weekly Arlt group meetings and discuss his/her work in detail with Prof Arlt, Dr Tennant and Dr Mueller in bi-weekly research meetings of the steroid sulfation sub-group. Finally, active participation in national or international endocrine conferences will be supported. The student role. The human cell line NCI-H295 derived from an adrenocortical carcinoma is a wellestablished model for adrenal function [5] with fetal characteristics. The student will subject NCI-H295 cells to extended periods of 3% oxygen mimicking the fetal oxygen tension. This will re-set their ‘normoxic’ set point, so that 3% becomes normoxia for them, as it is in utero. This adaptation will be monitored through regular sampling of the cell culture supernatant for analysis of steroid and steroid sulfate secretion and other metabolic markers of oxygenation (lactate, alanine). He or she will then model the change in oxygenation induced by birth by moving them to 12% oxygen, effectively “giving birth in the petri dish”. Thereafter the student will analyse the resulting changes in steroidogenesis (including expression changes of the sulfation enzymes SULT2A1 and PAPSS2), and alterations in cell viability, specifically through apoptosis. Mass spectrometric analysis of steroid sulfation is primarily carried out by specialist core facility staff, but the student will liaise with them and gain detailed insight into the methodologies, their pitfalls and opportunities. Data analysis, literature research and planning of further experimental procedures will be carried out by the student in close collaboration with Dr Mueller. 137 Lead Supervisor: Dr Stephen Young Contact Email: Telephone: S.P.Young@bham.ac.uk (0121) 371 3249 Co Supervisor: Dr Jane Falconer Project Title: How does the genetic variant of PTPN22 regulate macrophage metabolism and signalling? Department: Rheumatology Research group, Centre for translational inflammation Research, QEH Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Possibly Project Outline A genetic variant of the protein tyrosine phosphatase PTPN22, in which arginine 620 is changed to a tryptophan (R620W), is the most widely distributed polymorphism associated with a broad range of autoimmune disease. This includes rheumatoid arthritis, lupus and type I diabetes. We have shown that neutrophils expressing this variant are hyperactive and so may contribute to the inflammatory disease processes in autoimmunity. Preliminary data shows that this also affects the function of macrophages, and intriguingly also affects the metabolism of these cells. We have shown that metabolic profiling using NMR spectroscopy of serum and urine of rheumatoid arthritis patients can predict outcome early in disease and can also predict responses to therapeutic intervention. The source of the metabolites in the blood and urine may be partly from the inflamed synovium tissue in the joints but also from activated immune cells both locally and systemically. Macrophages are likely to contribute in a significant way to these metabolites and we hypothesise that macrophages expressing the PTPN22 will have a signature metabolic profile which will indicate significant differences in the function and may show how they contribute to driving the chronic immune inflammation associated with rheumatoid arthritis. References 1. 2. 3. 4. Bayley R, Kite KA, McGettrick HM, Smith JP, Kitas GD, Buckley CD, Young SP. The autoimmune-associated genetic variant PTPN22 R620W enhances neutrophil activation and function in rheumatoid arthritis patients and healthy individuals. Ann Rheum Dis. 2014;epub ahead. Young SP, Kapoor SR, Viant MR, Byrne JJ, Filer A, Buckley CD, Kitas GD, Raza K. The impact of inflammation on metabolomic profiles in patients with arthritis. Arthritis Rheum. 2013;65(8):2015-23. Kapoor SR, Filer A, Fitzpatrick M, Fisher BA, Taylor PC, Buckley CD, McInnes IB, Raza K, Young SP. Metabolic profiling predicts response to anti-TNFα therapy in patients with rheumatoid arthritis. Arthritis Rheum. 2013;65(6):1448-56. Fitzpatrick MA, Young SP. Metabolomics – A novel window into inflammatory disease. Swiss Med Wkly. 2013;143:w13743. How are you planning to ensure adequate supervision? Dr Young and Dr Falconer both actively work in the laboratory and would be available for direct supervision of the student. Dr Young supervises two further postdocs, two technicians and two graduate students working in this area all of whom could contribute to day-to-day supervision. The rheumatology group as a whole comprises around 50 people with two active research laboratories into which the student will be fully integrated. The student role. 138 In this project the student will differentiate blood monocytes into different types of macrophage and characterise their metabolic profiles using NMR-based analysis. This will be done with monocytes from individuals expressing wild-type and variant PTPN22 to determine how this phosphatase influences metabolism. The effect of the variant on signalling pathways regulated by PTPN22 will also be investigated using the analysis of calcium signalling and western blotting for phosphorylated targets of PTPN22. 139 Lead Supervisor: Dr. Kai-Michael Toellner Contact Email: Telephone: Email: k.m.toellner@bham.ac.uk Telephone: +44 (0)121 415 8687 Co Supervisor: Laura Garcia Ibanez Project Title: Migration Events from the Germinal Centre during Vaccine Responses Department: School of Immunity and Infection Will the project require a Home Office working with animals licence? Yes or No, can be acquired Is the Project Cancer related? No Project Outline Vaccination results in the production of high affinity protective antibody. The differentiation of high affinity memory B cells and plasma cells occurs in germinal centres (GC) in secondary lymphoid organs. Affinity maturation involves migration between the GC’s dark and light zones, and also migration of effector cells out of the GC. The signals that indicate to a B cell that it is time to differentiate and leave the GC are far from being fully understood. Chemokines, small secreted proteins that regulate leukocyte migration, are prime candidates to regulate this exit. It has been shown that chemokines have key roles in B cell differentiation and migration. We will evaluate the effect of the deficiency of chemokine receptor CCR7 on the exit of plasma cells and memory B cells from the spleen to other organs such as lymph nodes, blood and bone marrow. CCR7 binds to chemokines CCL19 and CCL21, produced by stromal cells in the vicinity of GCs. Mouse models deficient for CCR7 on B cells are available. Preliminary data shows that CCR7-deficient antigen-specific B cells form different memory B cell populations than CCR7-sufficient antigen-specific B cells but the relevance of these changes in memory B cell populations is unclear. The response of these memory B cells to subsequent antigen encounters may be affected and needs to be studied. The project will help to understand migration and differentiation of effector cells, especially of the different subsets of memory B cells, which may lead to new ways of manipulating antibody producing cells in vaccine responses. References Allen, C. D., T. Okada, J. G. Cyster (2007). “Germinal-center organization and cellular dynamics. ” Immunity 27(2): 190-202. Comerford, I., Y. Harata-Lee, M. D. Bunting, C. Gregor, E. E. Kara, S. R. McColl (2013). “A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptative immune system.” Cytokine Grow Factor Rev 24(3): 269-283. Zhang, Y., M. Meyer-Hermann, L. A. George,M.T. Figge, M. Khan, M. Goodall, S. P. Young, A. Reynolds, F. Falciani, A. Waisman, C. A. Notley, M.R. Ehrenstein, M. Kosko-Vilbois, K. M.Toellner (2013). “Germinal center B cells govern their own fate via antibody feedback.”J Exp Med 11;201(3): 457-464 140 How are you planning to ensure adequate supervision? All the experiments will be planned in regular meetings between student, PhD student co-supervising and main supervisor ensuring the logical progress of the project. Experiments performed in the lab will be directly supervised and guided by PhD student, who is expert in all techniques and procedures involved, and works on a closely related project with similar questions covering other chemokine receptors. Weekly meetings will be set up to ensure the satisfactory progress of the project and to deal with any difficulties that may have arisen. Further, our research group has weekly lab meetings, where problems are put in common with all the members of the lab and weekly journal clubs, where the most recent and relevant papers in the field are discussed. The student role. The student will receive full training in the following techniques: murine tissue preparation (spleen, lymph nodes, bone marrow and blood), flow cytometry, immunohistochemistry, light and fluorescence microscopy, ELISA and data analysis. The student will be directly involved in lab meetings, having the opportunity to discuss their work with the rest of the group for feedback and possible new ideas, in order to train in scientific discussion and project planning. Also, she/he will participate in journal clubs, giving her/him the possibility to improve presentation skills in a dissented environment. Procedures on animals can be carried out by the PhD student, and a Home Office license to perform procedures on animals can be acquired during the course of the project. 141 Lead Supervisor: Kai-Michael Toellner Contact Email: Telephone: k.m.toellner@bham.ac.uk Tel: 0121 415 8687 Co Supervisor: Yang Zhang Project Title: The gut, immune responses and the effects of aging Department: School of Immunity and Infection Will the project require a Home Office working with animals licence? Yes or No Is the Project Cancer related? No Project Outline Immunosenescence is a well known phenomenon of old age. Our ability to produce adequate responses to vaccination declines dramatically as we enter our 70s. How B cells are affected by aging remains a topic of study. Different B cell subsets have been shown to increase as we age (and are also associated with autoimmunity) (1.2). Our lab examined the contributions of the splenic environment compared to that of the intrinsic effects of aging on B cells. The data show that aged B cells produced more antibody and longer lived germinal centres when transferred into young hosts compared to young B cells. Further, the aged environment is greatly repressive to the immune responses of young B cells. The histological staining showed changes in B cell follicle structure in aged lymph nodes. Recently, a role for fibroblastic reticular cells (FRC) in the control of B cell homeostasis was shown in lymph nodes (3). Stromal cells also regulate production and migration of plasma cells. We have identified FRCs are in intimate contact with plasma cell differentiating from the germinal centre, and may regulate germinal centre derived plasma cell production. We hypothesize that changes of stromal cells in old age affect B cell homeostasis and immune responses after antigen challenge. The project will study these stromal cells before and after antigen challenge in gut, spleen, and other lymphoid tissues of young and aged mice, to investigate how ageing affects the distribution of stromal cells and their interactions with B cells. The project will involve immunohistochemistry, light and confocal microscopy and, time allowing, RT-PCR. References 1. Ademokun A et al, The ageing B cell population: composition and function, Biogerontology, 2010 11: 125-137 2. Scholz JL et al, A comparative review of aging and B cell function in mice and humans, Curr Opin Immunol, 2013, 25(4): 504-10 3. Cremasco V et al, B cell homeostasis and follicle confines are governed by fibroblastic reticular cells. Nat Immunol, 2014 15(10): 973-81 How are you planning to ensure adequate supervision? All the experiments will be planned in regular meetings between student, postdoctor co-supervising and main supervisor ensuring the logical progress of the project. Experiments performed in the lab will be directly supervised and guided by the postdoctor, who is expert in all techniques and procedures involved, and works on a closely related project with similar questions covering other chemokine receptors. Weekly meetings will be set up to ensure the satisfactory progress of the project and 142 to deal with any difficulties that may have arisen. Further, our research group has weekly lab meetings, where problems are put in common with all the members of the lab and weekly journal clubs, where the most recent and relevant papers in the field are discussed. The student role. The student will receive full training in the following techniques: murine tissue preparation (spleen, lymph nodes, bone marrow and blood), flow cytometry, immunohistochemistry, light and fluorescence microscopy, ELISA and data analysis. The student will be directly involved in lab meetings, having the opportunity to discuss their work with the rest of the group for feedback and possible new ideas, in order to train in scientific discussion and project planning. Also, she/he will participate in journal clubs, giving her/him the possibility to improve presentation skills in a dissented environment. Procedures on animals can be carried out by postdoctor, and a Home Office license to perform procedures on animals can be acquired during the course of the project. 143 Lead Supervisor: Kai-Michael Toellner Contact Email: Telephone: k.m.toellner@bham.ac.uk Tel: 012 4158687 Co Supervisor: Yang Zhang Project Title: Regulation of B cell responses specific for tumour expressed antigens Department: School of Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline More than 1/3 people in the UK will develop cancer, and more than 1/4 will die from it. Consequently there is great need for safer and more effective therapies. There is more and more evidence from clinical practice that antibodies are highly efficient drugs for the treatment of cancer. We recently published a new vaccine design that allows induction of high titres of antibody specific to tumour endothelium expressed antigen Robo4 (1). By chemically linking autoantigen to a foreign carrier protein to which pre-existing immunity is present, autoreactive B cells are able to recruit T cell help from primed carrier-specific T helper cells. Our published method used Robo4 as the tumour expressed autoantigen and chicken gamma globulin as the foreign carrier. We recently also have shown that coupling another tumour endothelium expressed antigen – CLEC14a – to the carrier tetanus toxoid (TT) results in antibody response with similarly efficiency are induced that confer tumour growth inhibition. CLEC14a, is highly present on the vasculature of many common human cancers (including liver, oesophageal, pancreatic, breast, bladder, ovarian) but is low/undetectable in healthy tissue (2). We hypothesize that target CLEC14a by using this type of vaccine can induce antibodies which specifically react with CLEC14a, and then damage tumour vessels and inhibit tumour growth without affecting normal, non-tumour vessels. The project will test the efficiency of the vaccine response when the tumour is already established, long term antibody production, and also test whether antibody response is inhibited by the presence of autoantigen. The project will involve fluorescent staining, Flow cytometry, ELISA, and, time allowing, RT-PCR. References 1. Zhuang, X., Ahmed, F., Zhang, Y., Ferguson, H. J., Steele, J. C., Steven, N. M., Nagy, Z., Heath, V. L., Toellner, K.-M. and Bicknell, R. (2014) Robo4 vaccines induce antibodies that retard tumor growth Angiogenesis. Available online 2. Mura, M., Swain, R. K., Zhuang, X., Vorschmitt, H., Reynolds, G., Durant, S., Beesley, J. F., Herbert, J. M., Sheldon, H., Andre, M., Sanderson, S., Glen, K., Luu, N. T., McGettrick, H. M., Antczak, P., Falciani, F., Nash, G. B., Nagy, Z. S. and Bicknell, R. (2012) Identification and angiogenic role of the novel tumor endothelial marker CLEC14A. Oncogene. 31, 293-305 144 How are you planning to ensure adequate supervision? All the experiments will be planned in regular meetings between student, postdoctor co-supervising and main supervisor ensuring the logical progress of the project. Experiments performed in the lab will be directly supervised and guided by the postdoctor, who is expert in all techniques and procedures involved, and works on a closely related project with similar questions covering other chemokine receptors. Weekly meetings will be set up to ensure the satisfactory progress of the project and to deal with any difficulties that may have arisen. Further, our research group has weekly lab meetings, where problems are put in common with all the members of the lab and weekly journal clubs, where the most recent and relevant papers in the field are discussed. The student role. The student will receive full training in the following techniques: murine tissue preparation (spleen, lymph nodes, bone marrow and blood), flow cytometry, immunohistochemistry, light and fluorescence microscopy, ELISA and data analysis. The student will be directly involved in lab meetings, having the opportunity to discuss their work with the rest of the group for feedback and possible new ideas, in order to train in scientific discussion and project planning. Also, she/he will participate in journal clubs, giving her/him the possibility to improve presentation skills in a dissented environment. Procedures on animals can be carried out by postdoctor, and a Home Office license to perform procedures on animals can be acquired during the course of the project. 145 Lead Supervisor: Dr Jianmin Zuo Contact Email: Telephone: J.Zuo@bham.ac.uk 0121-4144473 Co Supervisor: Dr Francesca Kinsella Project Title: Study of the role of NK Activation and Inhibition Receptors in the Graft versus Leukaemia effect and Graft versus Host Disease School Of Cancer Sciences Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? Yes Project Outline Stem Cell transplantation (SCT) is used widely in the management of haematological malignancies where its curative potential relies largely on the ‘graft versus leukaemia’ (GvL) effect that is mediated by the donor immune system. The mechanism of the GvL effect is uncertain but is believed to be mediated by donor-derived T and NK cells that recognise alloreactive proteins on the tumour target cell. As GvL is often associated with the detrimental effect of ‘graft versus host disease’(GvHD), a key aim within the SCT field is to selectively promote GvL whilst controlling GvHD. Work in our group has recently provided support for a role of the activatory NK receptor, NKG2D in GvL, by showing the impact of polymorphisms in its ligand ULBP6 on SCT outcomes. Recently, murine models have also provided evidence to suggest a role for other activatory receptors in modulating GvHD. We therefore propose to undertake a comprehensive study of NK cell activatory and inhibitory receptors in the SCT patients, including the stem cell bags and PBMCs post transplantation to assess their impact on GvL and GvHD. 1. NK cell activatory / inhibitory receptor expression before and after SCT will be studied using multi-colour flow-cytometry. NK subset cytokine production will be assessed ex vivo with intracellular cytokine staining, and their cytotoxic capacity assessed in vitro by their ability to kill target cells. 2. We propose to study the cohort patients to compare the phenotype of expanding T cells post-SCT and correlate with their NK phenotype. We will analyze the proportions of memory and naïve CD8 T cell populations, their proliferation (Ki67 146 staining), and cytokine production after SCT. A correlation will be sought between the CD8+ T cell phenotype, NK pheotype, and clinical outcome. 3. The Mixed Lymphocyte Reaction (MLR) is a functional assay which can measures the proliferative response of T lymphocytes from one individual to lymphocytes from another individual. It will be used to test the hypothesis of the regulation of T cell activation by NK cells. Importantly, by studying NK activation and inhibition receptors in the SCT will potentially guide strategies for obtaining better GvL while minimizing GvHD in SCT by manipulating NK population. References Antoun A, et al. (2012), 159(5), 589-598. The genotype of RAET1L (ULBP6), a ligand for human NKG2D (KLRK1), markedly influences the clinical outcome of allogeneic stem cell transplantation. British Journal of Haematology. Ghadially H, et al. (2014), 7(6), 1809–1814. NK Cell Receptor NKp46 Regulates Graft-versus-Host Disease. Cell Reports. How are you planning to ensure adequate supervision? Dr Zuo and Dr Kinsella will be guiding the student about the project and meeting regularly for discussion. Dr Zuo, Dr Kinsella and Mr Luke Maggs will help the student to master the lab-based technologies and also perform the day to day direct supervision. The student role. 1. In the lab, the student will carry out the lab work, such as PBMC separation, FACS staining, in vitro function assay (including NK cytotoxicity assay and Mixed Lymphocyte Reaction). 2. The student will attend the internal and external seminars of the department, will attend the group lab meetings and present their data, also will attend the journal club and discussion relevant paper to broad their scientific knowledge. 147 Lead Supervisor: Professor Hisham Mehanna Contact Email: Telephone: h.mehanna@bham.ac.uk 0121 414 6547 (Mrs Gemma Jones, PA to Hisham Mehanna) Dr Paul Nankivell, University of Birmingham Dr Mark Prince, Aston University Co Supervisor: Project Title: Developing a surgical robot for oropharyngeal cancer surgery. Department: Cancer Sciences Will the project require a Home Office working with animals licence? NO Is the Project Cancer related? Yes Project Outline The Institute of Head and Neck Studies and Education (InHANSE), led by Professor Hisham Mehanna, focuses on research into diseases of the head, neck and thyroid, and on the education of health professionals in the field. For head and neck cancer one of the most complex areas of management is the choice of primary treatment (surgery vs chemoradiotherapy) and this often relies on clinical factors (mainly clinical stage) and the therapeutic preference of the treating centre. Increasingly oropharyngeal cancer is being treated surgically with the advent of the intuitive surgical robot. However, this robot was designed for abdominal surgery and is not ideally suited for operating within the mouth and oropharynx. Our aim, therefore is to develop a robotic platform that is designed specifically for operating within the mouth and the oropharynx, especially the base of tongue and tonsil. We have started working on designing the robot. This is being done in collaboration with Dr Mark Prince from Aston University and Mr Chris Coulson from the Queen Elizabeth Hospital. Both have had experience in working on surgical robotic platforms in the head and neck. How are you planning to ensure adequate supervision? InHANSE is a team of over 15 researchers. This includes a clinical lecturer with an interest in the development of surgical devices – Paul Nankivell. The student will be supervised in their work both by Professor Mehanna and Dr Nankivell. Day to day supervision will be by Dr Nankivell, weekly updates to Professor Mehanna at the InHANSE weekly team meeting and monthly supervisory meetings with Professor Mehanna. The student role. 148 The student will undertake the following. 1. A review of the literature and the competitive landscape, examining approaches to operating in the oropharynx and existing robotic solutions. 2. Will carry out interviews with clinicians who have used the intuitive robot to explore what the current impediments are and how to improve it if possible. 3. Carry out an evaluation of dimensions and volumes of the oropharynx and mouth using 3D reconstructions of CT scans. 149 Lead Supervisor: Mark Webber Contact Email: Telephone: Co Supervisor: m.a.webber@bham.ac.uk 0121 414 2859 www.antimicrobialagentsresearchgroup.com Laura Piddock Project Title: Supercoiling and Superbugs Department: Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? No (Infection) Project Outline Antibiotic resistance is one of the great global health challenges of the 21st century and a problem which is raidly worsening with the emergence and spread of multidrug resistant pathogens and a dearth of new drugs in development. This has raised the real scenario of infections with untreatable pathogens and threatens an extraordinarily broad range of clinical activities where antibiotics are needed to both treat infection and provide prophylactic cover to allow complex surgery etc. Bacteria can employ a range of mechanisms to resist the actions of antibiotics, recently we have discovered that mutations within DNA gyrase which confer resistance to the powerful quinolone antibiotics by altering their target site also give a low level of resistance to many other drugs which do not target this enzyme. DNA gyrase controls how DNA is packaged (supercoiled) within the cell and changes in its activity alter chromosome structure and as a result expression of many other genes. We have found that gyrase mutants show up-regulation of stress response pathways which provide broad antimicrobial protection which we hypothesise to be due to a result of altered supercoiling. In this project we aim to take some of the stress responsive genes implicated in the antibiotic resistance phenotype and move them to new locations within the bacterial genome. We will then measure their expression (in response to mutation of DNA gyrase and in the presence and absence of quinolones) in order to determine whether local DNA supercoiling is key to production of a protective stress response. The project will contain a mixture of microbiology, molecular biology, antibiotic sensitivity testing and cellular permeability assays. 4. 5. 6. References Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock, LJ. Molecular mechanisms of antibiotic resistance. Nat Rev Micro. 2015 In press. Redgrave LS, Sutton SB, Webber MA, Piddock LJ. Fluoroquinolone resistance: mechanisms, impact on bacteria, and role in evolutionary success. Trends Microbiol. 2014 Aug;22(8):438-445. PMID:24842194 Clinically relevant mutant DNA gyrase alters supercoiling, changes the transcriptome and confers multi-drug resistance. Webber MA, Ricci V, Whitehead RN, Patel M, Fookes M, Ivens A, Piddock LJ. mBio. 2013. Jul 150 23;4(4). doi:pii:e00273-13. 23882012. 10.1128/mBio.00273-13. PubMed PMID: How are you planning to ensure adequate supervision? Students will meet weekly with Dr Webber and bi-weekly with both Dr Webber and Prof Piddock. They will be supervised daily by Dr Webber or other members of the antimicrobials research group which currently contains 15 full time members (www.antimicrobialagentsresearchgroup.com) ensuring appropriate cover will be available for student supervision. Webber has successfully supervised seven intercalating students in the last six years. The student role. The student will be responsible for performing and analysing experiments under the direction of Dr Webber and Prof Piddock and will be treated within the laboratory as any other member of the research team. The student will have responsibility for investigating the background of the project and developing a good awareness of the context and aims of the project 151 Lead Supervisor: Babu Naidu Contact Email: Telephone: b.naidu@bham.ac.uk Co Supervisor: Amy Kerr ( nee Bradley) Project Title: Amy.kerr@heartofengland.nhs.uk Department: CEM Will the project require a Home Office working with animals licence? No Is the Project Cancer related? yes Project Outline 5700 patients a year in the UK undergo major surgery to remove part of their lungs; primarily to cure cancer. A common post-operative complication is collapse or infection in the remaining lung (15% of cases). These complications are linked to increased risk of death, likelihood of admission into an intensive care unit and longer hospital stay. (1) We developed a comprehensive pulmonary rehabilitation programme (ROC- 2010) of an out-patient based programme consisting of exercise training, self-management education, nutritional and smoking cessation support and demonstrated in initial results improvement in complication and hospital readmission rates though data. (2) Lack of immediate access to pulmonary rehabilitation programmes across the country has hampered spread. There is thus a need to develop a service that can be delivered immediately at the convenience and in control of the patient. A tailored at home smart device ‘app’ based lung rehabilitation programme for lung surgery has already been developed but requires testing and refinement. (3) As with outpatient based pulmonary rehabilitation it aims to improve patient “fitness” for surgery, thus reducing post-surgical complications and enhancing recovery. This will improve long term patient centric outcomes such as quality of life and freedom from associated symptoms such as breathlessness. References 1. Agostini P, Cieslik H, Bishay E, Kalkat MS, Rajesh PB, Steyn RS, Naidu B The impact and risk factors for Post operative Pulmonary Complications following Lung Resection in current UK practice – Are there any modifiable factors?” Thorax. 2010 Sep;65(9):815-8. 2. Bradley A, Marshall A, Stonehewer L, Reaper L, Parker K, Bevan-Smith E, Jordan C, Gillies J, Agostini P, Bishay E, Kalkat M, Steyn R, Rajesh P, Dunn J, Naidu B. Pulmonary rehabilitation programme for patients undergoing curative lung cancer surgery. Eur J Cardiothorac Surg. 2013 Oct;44(4):e266-71 3. Scan for demo 152 How are you planning to ensure adequate supervision? The student will form part of the team of three research nurses, scientist and 2 clinical research fellows based at Heartlands hospital . We have a regular team meeting every two weeks followed by one to one sessions as required. The student will thus benefit from working in a supportive team environment The student role. The student will have two roles 1. The ROC programme is ongoing and since the initial publication collected data needs to be cleaned analysed written up and presented. 2. The ROC app has been developed but qualitative testing gathering feedback from patients carers and health care professionals is required. This will be collected into a report which will be disseminated at regional national and international meetings and will serve to refine the app prior to wider testing 153 Lead Supervisor: Dr Farhat Khanim Contact Email: Telephone: F.L.Khanim@bham.ac.uk Co Supervisor: Prof Chris Bunce, Prof Mark Drayson Project Title: Department: Can we identify microRNAs that are prognostic indicators of response to BaP therapy in AML/MDS patients? School of Biosciences and Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? YES Project Outline 3.3.4. Effect of BaP on microRNA (miRNA) and non-coding RNA (ncRNA) expression MiRNAs are a class of small noncoding RNA (∼22 nucleotides) that regulate gene expression by triggering message RNA (mRNA) degradation or inhibiting protein translation. Almost 1,000 miRNAs have been identified in the human genome and are thought to regulate 30% of the transcriptome. Not only have some miRNAs been demonstrated to have a role in the development of some types of haematological cancers, there is growing appreciation that heterogeneity amongst AML and CLL, and responses to therapy may be regulated at the level of miRNA activity. There are also direct links between miRNAs and the regulation of core metabolism and lipid metabolism. For example mitochondrial glutaminase (GLS), a key enzyme that converts glutamine to glutamate, which serves as a substrate in the TCA cycle for the production of ATP is regulated by miR-23a and miR-23b. MiRNAs are surprisingly stable and detectable in the circulation where they are proving to be a powerful diagnostic and prognostic tool in several diseases including AML, B cell lymphomas and myeloma. Our translational approach addresses acute myeloid leukaemia (AML), chronic lymphocytic leukaemia (CLL) and B- cell Non-Hodgkins lymphomas (B-NHL) including endemic Burkitt's lymphoma (eBL). These haematological malignancies share a common need for innovative therapies that display clinically significant anti-tumour activity without haematological toxicity. Our work began in AML and has led us to trialling a drug redeployment approach using combined Bezafibrate and medroxyprogesterone acetate (BaP). Our studies have also identified that the same drug combination has potential against CLL and B-NHL. It is therefore timely and necessary to investigate the role of miRNAs in mediating the antileukaemic effects of BaP and whether they can be used as prognostic indicators of response to BaP. We will perform microRNA microarrays on control vs BaP treated AML, CLL, and B-NHL cell lines and primary samples. These data will be integrated using bioinformatics approaches with responses to BaP treatment as measured using a range of cell-based and flow cytometric assays. Where targets of identified miRNAs are known we will investigate their modulated expression in response to treatments and their expression between responders and nonresponders (immunoblotting/QRTPCR). Where targets are unknown we will use lentiviral transgenic, proteomics and array approaches to identify proteins and mRNAs targets. References 154 Esquela-Kerscher, A. and F.J. Slack, Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer, 2006. 6(4): p. 259-69. Hayden, R.E., et al., Treatment of primary CLL cells with bezafibrate and medroxyprogesterone acetate induces apoptosis and represses the pro-proliferative signal of CD40-ligand, in part through increased 15dDelta12,14,PGJ2. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K, 2009. 23(2): p. 292-304. Khanim, F.L., et al., Combined bezafibrate and medroxyprogesterone acetate: Potential novel therapy for acute myeloid leukaemia. PLoS ONE, 2009. In Press. Murray, J.A., et al., Combined bezafibrate and medroxyprogesterone acetate have efficacy without haematological toxicity in elderly and relapsed acute myeloid leukaemia (AML). British journal of haematology, 2010. 149(1): p. 65-9 How are you planning to ensure adequate supervision? The student will be co-supervised by Dr Farhat Khanim, Prof Chris Bunce and Prof Mark Drayson. We will have regular meetings to discuss progress and to address any developments in the project. Daily laboratory supervision will be undertaken by Dr Farhat Khanim. Some of the techniques used in the project are undertaken in the Clinical Immunology Service run by Prof Mark Drayson. This will give the student a very useful insight into clinical laboratory techniques to compliment the cell and molecular techniques they will learn in the research laboratory. The student role. Clinical researchers are absolutely essential for successful translation of research findings into the clinic. Hence it is important that intercalating students have a positive research project experience and leave with the motivation and enthusiasm to continue with research. Hence, the student will work within the supportive network of a larger group of post-docs and students, will be encouraged to interact with them, and will be treated as a member of the research team. The project is organised such that the student will be very closely supervised during the first few weeks and semester. Once the student is confident in the lab and with the laboratory protocols, the students are given more independence to organise their own time and design the experiments. The student will be encouraged to read papers and develop their own ideas and hypotheses about the work and design their own experiments. They will also be encouraged to attend national and international meetings to present their work if a relevant meeting/conference comes up. This supervisory approach has been extremely successful with our previous students. Blair Merrick won a poster prize at the International Myeloma Workshop in Paris 2011 and Hannah Giles presented the work at a national student conference 2010 and won third prize for best oral presentation. Suzanne Raffles and Lauren Ferretti both won prizes and came top of the year for their intercalation projects (2012, 2013, respectively). Both Suzanne and Lauren also won prizes for best oral presentation on their projects at the International Doctors Research Symposium. Lauren also presented her work at a meeting in Vienna in 2013. Matt Fenton won best intercalation project prize, the Frank Kerr prize, and second place for the Claire Ripley award in 2014. Our goal is to give the students real experience of research and the importance of the interface between the lab and the clinic. 155 Lead Supervisor: Dr Farhat Khanim Contact Email: Telephone: F.L.Khanim@bham.ac.uk Co Supervisor: Prof Chris Bunce, Prof Mark Drayson Project Title: New use of old drugs in the treatment of myeloma; investigation of mechanisms of action against the malignant clone School of Biosciences and Immunity and Infection Department: Will the project require a Home Office working with animals licence? No Is the Project Cancer related? YES Project Outline Rationale Myeloma is a cancer of bone marrow plasma cells that impairs normal haemopoiesis and antibody production, destroys the skeleton and by secretion of M-protein causes renal failure. Treatment with high dose cytotoxic drugs and stem cell rescue improves survival. More recently biological therapies including thalidomide and its analogues and proteasome inhibitors have provided further improvement in survival but at a pharmaceutical cost of up to £50,000 per patient per year. Cures remain elusive and the disease kills 3,000 people per year in the UK. Translational research in Birmingham identified new molecular targets for a combination of two old drugs (progesterone and bezafibrate). As a result the drug combination is being tested as treatment for adult acute leukaemia and childhood Burkitt’s lymphoma. This strategy of redeployment of old drugs has been investigated by previous intercalating students who have identified a combination (VaN) of an anti-epileptic (valproate) and an anti-helminthic (niclosamide) with activities against myeloma cell lines. This very successful project has already resulted in 1 publication and a second manuscript is under preparation with all intercalating students as co-authors. The proposed project for 2015/2016 is a continuation of this work. We will be investigating the effect of VaN combination therapy on primary myeloma tumour cells both in vitro and also using a mouse model in collaboration with a group in Sheffield. The mouse model will be used to study the effect of the drugs on myeloma induced lytic bone lesions, cytokine secretion and disease progression. A previous student identified that VaN modifies the “acetylome” (acetylated proteins) in myeloma cells. We will continue these studies looking at the effect of VaN therapy on the acetylome of myeloma cells using a combination of molecular, cellular and biochemical approaches. The ultimate aim is to generate enough data to initiate phase II clinical trials of VaN therapy in myeloma. References Khanim, F.L., et al., Redeployment-based drug screening identifies the anti-helminthic niclosamide as anti-myeloma therapy that also reduces free light chain production. Blood cancer journal, 2011. 1(10): p. 21. How are you planning to ensure adequate supervision? 156 The student will be co-supervised by Dr Farhat Khanim, Prof Chris Bunce and Prof Mark Drayson. We will have regular meetings to discuss progress and to address any developments in the project. Daily laboratory supervision will be undertaken by Dr Farhat Khanim. Some of the techniques used in the project are undertaken in the Clinical Immunology Service run by Prof Mark Drayson. This will give the student a very useful insight into clinical laboratory techniques to compliment the cell and molecular techniques they will learn in the research laboratory. The student role. Clinical researchers are absolutely essential for successful translation of research findings into the clinic. Hence it is important that intercalating students have a positive research project experience and leave with the motivation and enthusiasm to continue with research. Hence, the student will work within the supportive network of a larger group of post-docs and students, will be encouraged to interact with them, and will be treated as a member of the research team. The project is organised such that the student will be very closely supervised during the first few weeks and semester. Once the student is confident in the lab and with the laboratory protocols, the students are given more independence to organise their own time and design the experiments. The student will be encouraged to read papers and develop their own ideas and hypotheses about the work and design their own experiments. They will also be encouraged to attend national and international meetings to present their work if a relevant meeting/conference comes up. This supervisory approach has been extremely successful with our previous students. Blair Merrick won a poster prize at the International Myeloma Workshop in Paris 2011 and Hannah Giles presented the work at a national student conference 2010 and won third prize for best oral presentation. Suzanne Raffles and Lauren Ferretti both won prizes and came top of the year for their intercalation projects (2012, 2013, respectively). Both Suzanne and Lauren also won prizes for best oral presentation on their projects at the International Doctors Research Symposium. Lauren also presented her work at a meeting in Vienna in 2013. Matt Fenton won best intercalation project prize, the Frank Kerr prize, and second place for the Claire Ripley award in 2014. Our goal is to give the students real experience of research and the importance of the interface between the lab and the clinic. 157 Lead Supervisor: Dr Farhat Khanim Contact Email: Telephone: F.L.Khanim@bham.ac.uk Co Supervisor: Prof Chris Bunce, Prof Mark Drayson Project Title: Can bacterial NDK proteins regulate human haemopoiesis and leukaemic progression? Department: School of Biosciences and Immunity and Infection Will the project require a Home Office working with animals licence? No Is the Project Cancer related? YES, Infection related also Project Outline Rationale We have shown that the human nucleotide diphosphate kinase (NDK) protein, NM23-H1, is able to promote survival and induce proliferation of human haemopoietic stem cells, both normal and leukaemic (Lilly et al, 2011, Manuscript in preparation). NDKs are highly evolutionarily conserved and are also expressed by bacteria. Based on our previous data with human NDK (NM23-H1) and the fact that bacterial infection is a common clinical feature of leukaemia’s, also myelodysplastic syndromes (MDS), and is often the cause of mortality in these patients, we hypothesised that bacterial NDK proteins generated during infection may promote progression of the leukaemia or MDS. In collaboration with Mark Webber (IMI), we have cloned NDK ORFs from 2 gram-positive and 2 gram-negative bacteria and produced recombinant HIS-tagged protein. Although we need to treat more samples, preliminary data shows clearly that the bacterial NDK proteins are able to induce similar effects on human umbilical blood derived haemopoietic stem cells as recombinant human NDK (NM23-H1). This work has major implications for how bacterial infections are managed in leukaemia/MDS patients but also raises very interesting questions about the influence of bacteria on the evolution of the haemopoietic system and haemopoietic stem cells. The proposed project for 2015/2016 is a continuation of this work. We will be investigating the effect of human and bacterial NDK proteins on adult normal donor derived haemopoietic stem cells (HSCs) and leukaemic cells. We will look at the effect on cell proliferation as measured using cellular and flow cytometry based assays. Luminex analysis will be used to dissect the influence of human and bacterial NDK proteins on cytokine and growth factor secretion. We will use a combination of molecular and proteomics strategies to identify the receptor for bacterial NDKs on HSCs and leukaemia cells. We will also investigate the possibility of generating antibodies specific for the bacterial NDKs so that we can develop an ELISA that can be used on patient sera to monitor bacterial NDK levels in MDS and leukaemia patients. References Lilly A.J., Khanim F.L., Hayden R.E. et al. Nm23-h1 indirectly promotes the survival of acute myeloid leukemia blast cells by binding to more mature components of the leukemic clone. Cancer research 71, 1177-1186, doi:10.1158/0008-5472.CAN-101704 Niitsu N., Okabe-Kado J., Okamoto M. et al. Serum nm23-H1 protein as a prognostic factor in aggressive non-Hodgkin lymphoma. Blood 97, 1202-1210 (2001) Chopra P., Singh A., Koul A. et al. Cytotoxic activity of nucleoside diphosphate kinase secreted from Mycobacterium tuberculosis. Eur J Biochem 270, 625-634, 158 doi:3402 [pii] (2003). How are you planning to ensure adequate supervision? The student will be co-supervised by Dr Farhat Khanim, Prof Chris Bunce and Prof Mark Drayson. We will have regular meetings to discuss progress and to address any developments in the project. Daily laboratory supervision will be undertaken by Dr Farhat Khanim. Some of the techniques used in the project are undertaken in the Clinical Immunology Service run by Prof Mark Drayson. This will give the student a very useful insight into clinical laboratory techniques to compliment the cell and molecular techniques they will learn in the research laboratory. The student role. Clinical researchers are absolutely essential for successful translation of research findings into the clinic. Hence it is important that intercalating students have a positive research project experience and leave with the motivation and enthusiasm to continue with research. Hence, the student will work within the supportive network of a larger group of post-docs and students, will be encouraged to interact with them, and will be treated as a member of the research team. The project is organised such that the student will be very closely supervised during the first few weeks and semester. Once the student is confident in the lab and with the laboratory protocols, the students are given more independence to organise their own time and design the experiments. The student will be encouraged to read papers and develop their own ideas and hypotheses about the work and design their own experiments. They will also be encouraged to attend national and international meetings to present their work if a relevant meeting/conference comes up. This supervisory approach has been extremely successful with our previous students. Blair Merrick won a poster prize at the International Myeloma Workshop in Paris 2011 and Hannah Giles presented the work at a national student conference 2010 and won third prize for best oral presentation. Suzanne Raffles and Lauren Ferretti both won prizes and came top of the year for their intercalation projects (2012, 2013, respectively). Both Suzanne and Lauren also won prizes for best oral presentation on their projects at the International Doctors Research Symposium. Lauren also presented her work at a meeting in Vienna in 2013. Matt Fenton won best intercalation project prize, the Frank Kerr prize, and second place for the Claire Ripley award in 2014. Our goal is to give the students real experience of research and the importance of the interface between the lab and the clinic. 159
© Copyright 2025