Synthesis, spectroscopic characterization and vulcanization activity
Available online at www.sciencedirect.com
EUROPEAN
POLYMER
JOURNAL
European Polymer Journal 43 (2007) 4706–4711
www.elsevier.com/locate/europolj
Synthesis, spectroscopic characterization and vulcanization
activity of a new compound containing the anion
bis(4-methylphenylsulfonyldithiocarbimato)zincate(II)
Roberta M. Mariano a, Marcelo R.L. Oliveira b, Mayura M.M. Rubinger b,
Leila L.Y. Visconte a,*
a
Instituto de Macromole´culas Professora Eloisa Mano/Universidade Federal do Rio de Janeiro, P.O. Box 68525, Rio de Janeiro, RJ, Brazil
b
Departamento de Quı´mica, Universidade Federal de Vic¸osa, Vic¸osa MG, CEP 36570-000, Brazil
Received 20 April 2007; received in revised form 6 July 2007; accepted 28 August 2007
Available online 2 September 2007
Abstract
A new compound of the formula: (Bu4N)2[Zn(4-CH3C6H4SO2N@CS2)2] (Bu4N = tetrabutylammonium cation) was
obtained by the reaction of CH3C6H4SO2N@CS2K2 Æ 2H2O with zinc(II) acetate dihydrate and tetrabutylammonium bromide in dimethylformamide. The elemental analyses of C, H, N and Zn are consistent with the proposed formula. The IR
data point to the formation of a zinc(II)–bis(dithiocarbimato) complex. The 1H NMR and 13C NMR spectra showed the
expected signals for the tetrabutylammonium cation and the dithiocarbimato moiety. This compound was evaluated as for
the vulcanization activity and compared to the commercial accelerators CBS, MBTS and TMTD. These studies showed
that the zinc(II)–ditiocarbimates are a new class of rubber vulcanization accelerators, worth of further investigation.
Ó 2007 Elsevier Ltd. All rights reserved.
Keywords: Dithiocarbimates; Zinc complexes; Vulcanization
1. Introduction
Zinc(II)–bis(dithiocarbamato) complexes are used
worldwide in rubber vulcanization [1–6] and are
known as ultra-accelerators due to their extremely
rapid acceleration properties [2]. Vulcanizing
mixtures containing zinc(II)–tris(dithiocarbamato)
complexes of the general formula: [Zn(R2NCS2)3]
were found to be more accelerated than mixtures
*
Corresponding author. Tel.: +55 21 2562 7204; fax: +55 21
2270 1317.
E-mail address: lyv@ima.ufrj.br (L.L.Y. Visconte).
containing zinc(II)–bis(dithiocarbamato) complexes
of the general formula [Zn(R2NCS2)2] [2,6]. The
anionic species are more soluble in organic solvents
[6] and stronger nucleophiles than the parent
bis(dithiocarbamato) neutral complexes [2]. These
facts can be responsible for their greater activity.
Our interest in the research on dithiocarbimatometal-complexes is based on the similarities these
compounds show when compared with the dithiocarbamate complexes, mainly as long as the presence of the Zn(S2C@NR)2 moiety in the former
structures is concerned. However, small differences
between these structures can be very important.
0014-3057/$ - see front matter Ó 2007 Elsevier Ltd. All rights reserved.
doi:10.1016/j.eurpolymj.2007.08.013
R.M. Mariano et al. / European Polymer Journal 43 (2007) 4706–4711
4707
CH3C6H4SO2N@CS2K2 Æ 2H2O was prepared in
dimethylformamide from the 4-toluenessulfonamide, CS2 and KOH, as described in the literature
[7]. Microanalyses for C, H and N were obtained
from a Perkin-Elmer 2400 CHN elemental analyzer.
Zinc was analysed by atomic absorption with a
Hitachi Z-8200 Atomic Absorption Spectrophotometer. The IR spectra were recorded with a Perkin-Elmer 283 B infrared spectrophotometer using
CsI pellets. The 1H (200 MHz) and 13C (50 MHz)
NMR spectra were recorded with a Bruker DPX200-AVANCE spectrophotometer in CDCl3 with
TMS as internal standard.
For example, the zinc(II)-bis(dithiocarbimato) complexes are, necessarily, anionic species. So, the
improvement and/or modulation of the vulcanization activity is an interesting possibility, which can
be accomplished either by modifying the solubility
of the complexes salts with the use of different cations or different R groups on the dithiocarbimate
structures, or by using active counter ions.
In this work the vulcanization efficiency of a new
compound containing the anionic complex bis(4methylphenylsulfonildithiocarbimato)zincate(II)
with the formula (Bu4N)2[Zn(4-CH3C6H4SO2N@
CS2)2] (Bu4N = tetrabuthylammonium cation),
ZNIBU, was investigated. This compound was
obtained by the reaction of Zn(CH3COO)2 Æ 2H2O
with potassium 4-methylphenylsulfonyldithiocarbimate dihydrate and tetrabutylammonium bromide
and was characterized by IR, 1H and 13C NMR
spectroscopies, and C, H, N and Zn elemental analyses. Standard compositions of natural rubber
(NR), unfilled (gum) or filled with carbon black
were studied as for their rheometric parameters.
For comparison purposes, the commercial accelerators CBS (N-cyclohexylbenzothiazole sulfenamide),
MBTS (dibenzothiazole disulfide) and TMTD
(tetramethylthiuram disulfide) were also used.
2.2. Synthesis
The synthesis was performed according to Fig. 1.
Zinc(II) acetate dihydrate (74.41 g, 0.34 mol)
and tetrabutylammonium bromide (219.83 g,
0.68 mol) were added to a solution of potassium
4-methylphenylsulfonyldithiocarbimate dihydrate
(244.77 g, 0.68 mol) in methanol (300 mL) and
water (900 mL). The mixture was stirred at room
temperature for 1 h and the yellowish solid product
was filtered, washed with distilled water (4 L), ethanol (300 mL) and diethyl ether (150 mL) and dried
under reduced pressure, yielding (Bu4N)2[Zn(4CH3C6H4SO2N@CS2)2] (327.44 g, 0.31 mol).
Tetrabutylammonium bis(4-methylphenylsulfonyldithiocarbimato)zincate(II), (Bu4N)2[Zn(4-CH3C6H4SO2N@CS2)2]: Elemental analyses: Found
(calculated for C48H86N4O4S6Zn): C, 55.24
(55.38); H, 8.06 (8.33); N, 5.44 (5.38); Zn, 6.20
(6.28) %. M.p. (°C): 161.8-162.2. IR (most intense
bands) (cm 1): 1372 m(C@N); 1266 mass(SO2); 1136
msym(SO2); 939 mass(CS2) and 312 m(ZnS). 1H NMR
(d) J(Hz): 7.83 (d, 4H, J = 8.2, H2 and H6); 7.17
2. Experimental
2.1. Methods and materials
The solvents were purchased from Merck and
used without further purification. The chemicals
sulfonamide, zinc acetate dihydrate and tetrabutylammonium bromide were purchased from Aldrich.
Carbon disulfide and potassium hydroxide were
purchased from Vetec. The compound 4-
2 p-CH3C6H4SO2N=CS2K2.2H2O + Zn(CH3COO)2.2H2O + 2 Bu4NBr
CH3OH - CH3COOK
- KBr
H 2O
O
(Bu4N)2 H3C
S
O
N C
S
S
Zn
O
S
S
C N
S
O
ZNIBU
Fig. 1. Scheme for ZNIBU preparation.
CH3
4708
R.M. Mariano et al. / European Polymer Journal 43 (2007) 4706–4711
(d, 4H, J = 8.1, H3 and H5); 2.34 (s, 6H, CH3); Tetrabutylammonium cation: 3.24, (m, 16H, CH2N);
1.60–1.50 (m, 16 H, CH2); 1.46–1.26 (m, 16 H,
CH2); 0.95 (t, 24 H, J = 7.0, CH3). 13C NMR (d):
208.50 (N@CS2); 141.00 (C1); 127.72 (C2 and C6);
128.32 (C3 and C5); 140.14 (C4); 21.41 (CH3); Tetrabutylammonium cation signals: 58.60 (CH2N);
23.93 (CH2); 19.67 (CH2); 13.73 (CH3).
2.3. Vulcanization
Natural rubber was compounded following the
formulation (in phr): natural rubber, NR (100),
stearic acid (2.5), zinc oxide (3.5), sulfur (2.5), carbon black (0 or 20), aminox (2.0), accelerator (0.8
or 1.2). Natural rubber, Mooney viscosity ML
1 + 4(100 °C) = 74.8, was supplied by Sociedade
Michelin de Participac¸o˜es, Indu´stria e Come´rcio
Ltda; the other ingredients were of grades commonly used in the industry.
The mixture was carried out in a roll mill with
1:1.25 friccion rate, at room temperature. Rheometric parameters were determined in an oscillating
disk rheometer from Tecnologia Industrial, at
150 °C and 1° arc, according to ASTM D 2084.
The mixes were vulcanized and molded into sheets
in a hydraulic press operating at 150 °C and
3.0 MPa. From the sheets, specimens for stress
strength (ASTM D412) and tear resistance (ASTM
D 624) tests were cut.
3. Results and discussion
The compound here studied is quite stable at
the ambient conditions. It is slightly soluble in
water, methanol and ethanol, and is soluble in
chloroform and dichloromethane. A strong band
at 1372 cm 1 observed in its vibrational spectrum
was assigned to the mC@N vibration. The massCS2
was observed at higher frequency in the spectrum
of the parent potassium salt of dithiocarbimate
(955 cm 1) [8] than in the spectrum of the tetrabutylammonium bis(4-methylphenylsulfonyldithiocarbimato)zincate(II)
(939 cm 1),
confirming
the
complexation [9]. The spectrum of the title compound also shows the expected medium band in
the 312 cm 1, assigned to the Zn–S stretching
vibration, indicating a bidentade coordination by
two sulfur atoms of the dithiocarbimato ligand
[10,11]. The 1H NMR spectrum showed all the signals for the hydrogen atoms of the tetrabutylammonium cation. The remaining signals could be
assigned to the CH3 group of the aromatic moiety
and to the aromatic hydrogen atoms. The integration curves on the 1H NMR spectrum were consistent with a 2:1 proportion between the
tetrabutylammonium cation and the complex
anion. The 13C NMR spectrum showed the six
expected signals due to the dithiocarbimato
moiety. The N@CS2 (C1) signal is shifted in the
spectrum of the compound to higher field if
Table 1
Rheometric parameters for NR mixes compounded with ZNIBU, CBS, MBTS or TMTD as the accelerator
Accelerator
Accelerator amount
(phr)
ZNIBU
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
CBS
MBTS
TMTD
Carbon black
(phr)
0
20
0
20
0
20
0
20
Minimum torque, Ml
(dN m)
Maximum torque, Mh
(dN m)
(Mh–
Ml)
t90
(min)
4.85
5.53
6.66
5.31
2.82
2.93
4.29
6.21
3.39
6.00
6.10
6.32
3.05
4.63
5.31
6.55
25.29
25.06
31.72
32.06
25.50
27.09
30.60
34.00
21.34
23.03
26.42
27.54
25.49
27.55
29.12
34.40
20.44
19.53
25.06
26.75
22.68
24.16
26.31
27.79
17.95
17.03
20.32
21.22
22.44
22.92
23.81
27.85
35.4
30.0
29.4
21.0
11.6
8.7
11.4
8.3
12.0
7.6
12,0
10.2
4.8
3.6
4.4
3.8
t90: optimim vulcanization time; CBS: N-cyclohexyl-2-benzothiazol-2-sulfenamide; MBTS: benzothiazol disulfide; TMTD: tetramethylthiuram disulfide.
R.M. Mariano et al. / European Polymer Journal 43 (2007) 4706–4711
compared to the spectrum of the ligand (225.19 d)
[8] due to the complexation by two sulfur atoms.
The spectroscopic data for the compound
here studied are very similar to those observed for
the analogous compound tetraphenylphosphonium
bis(4-methylphenylsulfonyldithiocarbimato)
zincate(II) [9] and tetraphenylphosphonium bis(methylsulfonyldithiocarbimato)zincate(II) [10] that have
a distorted tetrahedral configuration around the zinc(II) cation due to the bidentate chelation by two
sulfur atoms of the dithiocarbimato ligands.
The efficiency of ZNIBU as an accelerator for
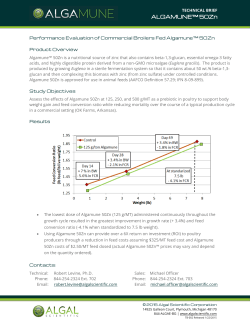
natural rubber vulcanization was investigated by
rheometry. Table 1 shows data from rheometric
curves obtained for ZNIBU as well as for CBS,
MBTS and TMTD as accelerators. These three
4709
compounds were chosen because they pertain to different classes of accelerators and therefore they will
have different influences on the rubber towards vulcanization [12–14]. Consequently, characteristics
such as cure rate, safety scorch, number and type
of the crosslinks formed will be dictated by the
choice of the accelerator.
From data of t90 in Table 1 it can be seen that vulcanization with ZNIBU proceeds at slower rates than
with any of the other compounds. An acceleration is
accomplished by increasing the amount of ZNIBU,
specially in the presence of carbon black. In all cases,
the addition of this filler has a positive effect on
(Mh–Ml), which is related to the degree of crosslinking, particularly when using ZNIBU as the
accelerator. For this compound, the values of
Fig. 2. Rheometric curves for NR compounded with 0.8 phr of different accelerators 1: ZNIBU; 2: CBS; 3: MBTS; 4: TMTD.
Fig. 3. Rheometric curves for NR compounded with ZNIBU. 1: 0.8 phr ZNIBU; unfilled 2: 0.8 phr ZNIBU; filled 3: 1.2 phr ZNIBU;
unfilled 4: 1.2 phr ZNIBU; filled.
4710
R.M. Mariano et al. / European Polymer Journal 43 (2007) 4706–4711
(Mh–Ml) are almost of the same magnitude as those
obtained for CBS, one of the most used accelerators
for NR.
The rheometric curves recorded during vulcanization of the compounds in the presence of 0.8 phr
of accelerator are shown in Fig. 2.
These curves show the reversion tendency of NR
under continuous heating. In the presence of any of
the commercial accelerators, torque reaches its max-
a
CBS
TMTD
ZNIBU
MBTS
Stress strength (MPa)
25
20
15
10
5
0
0
200
400
600
800
Elongation (%)
Stress strength (MPa)
b 25
20
15
10
5
0
0
100
200
300
400
500
600
700
800
Elongation (%)
Stress strength (MPa)
c 25
20
Table 2
Tear resistance of NR compounds vulcanized with different
accelerators
15
10
5
100
200
300
400
500
600
700
800
Elongation (%)
Stress strength (MPa)
Accelerator
Accelerator
amount (phr)
ZNIBU
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0.8
1.2
0
0
d
imun value and then starts to decrease. This is a well
known NR characteristic and is the result of degradation brought about by oxidation of the remaining
double bonds in the rubber main chain. Interestingly this seems not happen in the presence of
ZNIBU, during the duration of the experiment.
Upon incorporation of a larger amount of accelerator, 1.2 phr, the same trend is observed in the
unfilled compounds (curves 1 and 3 in Fig. 3), and
still no reversion tendency is observed. This begins
to show up at 1.2 phr of ZNIBU in the filled
composite.
Fig. 4 shows data of stress strength for the compounds in the presence of the different accelerators.
From the curves in Fig. 3a it can be seen that
ZNIBU and CBS have similar behavior and present
the highest results. In the presence of carbon black
(Fig. 3b) these two accelerators give rise to compounds which still behave similarly.
An increment in these accelerators content for
the unfilled compounds (Fig. 3c) leads to a slight
decrease in elongation, a small increase in 300%
modulus but the stress resistance remains practically
unchanged. In the presence of carbon black,
ZNIBU, CBS and MBTS give results which are very
close to each other. However, considering ZNIBU
alone, the addition of this filler did not bring any
improvement in the property.
For the unfilled compounds vulcanized with
0.8 phr of accelerator, shown in Table 2, the highest
values of tear resistance are given by ZNIBU and
CBS. On increasing the amount of these two
25
CBS
20
15
10
MBTS
5
0
0
100
200
300
400
500
600
700
800
Elongation (%)
Fig. 4. Stress resistance of the filled and unfilled compounds
vulcanized with different amounts of accelerators: (a) 0.8 phr,
unfilled; (b) 0.8 phr, filled; (c) 1.2 phr, unfilled; (d) 1.2 phr, filled.
TMTD
Carbon
black (phr)
0
20
0
20
0
20
0
20
Tear resistance
(kN/m)
34.8
28.0
36.7
37.1
34.7
33.9
39.1
40.0
30.9
33.1
33.7
34.4
30.2
31.2
38.4
34.6
R.M. Mariano et al. / European Polymer Journal 43 (2007) 4706–4711
accelerators, the property worsens in opposition to
what happens with the other compounds. The positive effect of carbon black is seen in all cases and concerning this property, ZNIBU behaves very much in
the same way as the commercial accelerators.
4. Conclusion
A new salt of bis(4-methylphenylsulfonyldithiocarbimato)zincate(II) complex was prepared (see
Fig. 1). The tetrabutylammonium salt was isolated
and characterized by IR, 1H, and 13C NMR and
by elemental analyses. The addition of the complex
to a standard natural rubber formulation has shown
that it behaves as a slow accelerator for vulcanization. Nevertheless, as far as mechanical properties,
stress and tear resistances, are concerned, NR compounds formulated with ZNIBU give values which
are of the same magnitude as those found for NR
compounds vulcanized in the presence of three of
the most used commercial accelerators, CBS, MBTS
and TMTD.
By increasing the amount of ZNIBU from 0.8 to
1.2 phr, stress strength of the unfilled compounds
did no change and decreased in the presence of carbon black. Concerning the optimum vulcanization
time, t90, larger ZNIBU contents lead to reduced
values, but still too long when compared to the commercial accelerators.
ZNIBU is the first example of a novel class of
rubber vulcanization accelerators. Analogous compounds are being prepared to extend the studies
on their vulcanization activities.
Acknowledgement
This work has been supported by the brazilian
research council, CNPq.
References
[1] Coucouvanis D. The chemistry of the dithioacid and 1,1dithiolate complexes. Prog Inorg Chem 1969;11:233.
4711
[2] Nieuwenhuizen PJ, Reedijk J, van Duin M, McGill WJ.
Thiuram- and dithiocarbamate-accelerated sulfur vulcanization from the chemist’s perspective; methods, materials and
mechanisms reviewed. Rubber Chem Technol 1997;70:368.
[3] Hogarth G. Transition metal dithiocarbamates: 1978–2003.
Prog Inorg Chem 2005;53:71.
[4] Nieuwenhuizen JP, Timal S, Haasnoot JG, Spek AL,
Reedijk J. Zinc(II)-catalyzed disproportionation in rubber:
The mechanism of sulfur vulcanization revisited. Chem Eur
J 1997;3:1846.
[5] Nieuwenhuizen PJ, Ehlers AW, Haasnoot JG, Janse SR,
Reedijk J, Baerends EJ. The mechanism of zinc(II)-dithiocarbamate-accelerated vulcanization uncovered; theoretical
and experimental evidence. J Am Chem Soc 1999;121:163.
[6] MaCleverty JA, Spencer N, Bailey NA, Shackleton SL.
Aspects of the inorganic chemistry of rubber vulcanization
Part 1. Reactions of zinc bis(dithiocarbamates) and bis(benzothiazole-2-thiolates) with carboxylates, and the structure
of [NBun 4 ] [{Zn(S2-CNMe2)2}2 (l-OCOMe)]. J Chem Soc
Dalton Trans 1980;10:1939.
[7] Hartke K. Darstellung von sulfonylisothiocyanaten. Arch
Pharm 1966;299:164.
[8] Oliveira MRL, De Bellis VM. Preparation of novel
cobalt(III) complexes with dithiocarbimates derived from
sulfonamides. Trans Met Chem 1999;24:127.
[9] Perpe´tuo GJ, Oliveira MRL, Janczak J, Vieira HP, Amaral
FF, De Bellis VM. Syntheses, crystal structure and spectroscopic characterization of novel N–R-sulfonyldithiocarbimate zinc(II) complexes. Polyhedron 2003;22:3355.
[10] Oliveira MRL, Perpe´tuo GJ, Janczak J, Rubinger MMM.
Synthesis, structural and spectroscopic characterization of
novel zinc(II) complexes with N-methylsulfonyldithiocarbimato and N-methylsulfonyltrithiocarbimato ligands. Polyhedron 2007;26:163.
[11] Nakamoto K. Infrared and Raman of inorganic and
coordination compounds. New York: John Wiley & Sons,
Inc.; 1978. p. 339.
[12] Travas-Sejdic J, Jelencic J, Bravar M, Fro¨be Z. Characterization of the natural rubber vulcanizates obtained by
different accelerators. Eur Polym J 1996;32(12):1395.
[13] da Costa HM, Visconte LLY, Nunes RCR, Furtado CG.
Aspectos histo´ricos da vulcanizac¸a˜o. Polı´meros: Cieˆncia e
Tecnologia 2003;13(2):125.
[14] da Costa HM, Visconte LLY, Nunes RCR, Furtado CG.
Rice husk ash filled natural rubber compounds III. The role
of metal oxides on the kinetics of sulfur vulcanization. J
Appl Polym Sci 2003;90:1519.
© Copyright 2025