FIG. / g
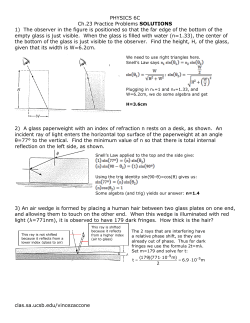
July 16, 1968 3,393,060 G. E. BLAIR ETAL METHOD OF CHANGING THE CONDUCTIVITY OF CERAMIC MATERIALS Original Filed March 18, 1963 2 Sheets-Sheet 1 5 FIG. / g w‘ z a ‘ wt as L v; o5_ Ba (PO, ,3; FIG. 3 5' " '°" I 2' 0 % ~- I o 2 S w‘ '- | won IO’ - 1 .ao; 1 .053 l I‘ .004 we TEMPERATURE I/oK >|c cola 1 now | ' l 9 .90! .°°| TEMPERATURE l/oK INVENTORS FIG. 2 um» E- mm DAVID P. HAMBLEII ROBE!!! A. WEIDEL BY ATTORKEYS July 16, 1968 G. E. BLAIR ETAL 3,393,060 METHOD OF CHANGING THE CONDUCTIVITY OF CERAMIC MATERIALS Original Filed March 18, 1963 2 Sheets-Sheé‘t 2 ‘o w 50' Z 9 U) Q I _ (D Z :1‘ k- . I 1° ' IO I a z. I I 0 ¢ 5 WAVE LENGTH- MICRONS CM.OHM- o I; 310 0'0 11L TIME - MINUTES zie aid INVENTORS GERALD E- FIG. 4 BLAIR DAVID R BAMBI-EN ROBERT A. WEIDEL ATTORNE 8 1 United States Patent O cice 3,393,060 Patented July 16, 1968 1 2 3,393,060 vanadium oxide potassium metaphosphate glass; FIG. 3 is a graphical representation showing the elfect tionship between speci?c resistivity and temperature for a METHOD OF CHANGING THE CONDUCTIVITY OF ‘CERAMIC MATERIALS Gerald E. Blair, Galita, Calif., and David P. Hamblen, Gates, and Robert A. Weidel, Webster, N.Y., assignors on speci?c resistivity when the vanadium oxide potassium metaphosphate glass is held at various temperatures for ?ve hours; annealed and measured at 40° C.; FIG. 4 is a graphical representation showing the varia tion in speci?c resistivity when samples of vanadium meta phosphate glass are held at 310° C. for various times, to Bausch 8: Lomb Incorporated, Rochester, N.Y., a corporation of New York Continuation of application Ser. No. 265,636, Mar. 18, 1963. This application Oct. 13, 1966, Ser. No. 586,573 2 Claims. (Cl. 65-33) 10 then are annealed and measured at 40° C. and, FIG. 5 is a graphical representation showing the infra red transmission of one sample of vanadium phosphate glass at various temperatures. ABSTRACT OF THE DISCLOSURE The novel treatment may be defined as promoting nu A method is disclosed vfor reducing the speci?c resis tivity of a glass composition including the major portion 15 cleation and subsequently crystallization in a vitreous ma terial, frequently called devitri?cation. It is presently pre of vanadium pentoxide and a minor portion of numerous ferred to treat the materials ‘by subjecting them to heat, metal phosphates. The glass substrate is heated in a tem i.e. to a temperature between the liquidus temperature perature range between the transition temperature and and the transformation temperature for a period of ap the liquidus temperature for a period of time sut?cient to cause crystallization. The crystallization effectively re 20 proximately 1/2 hour. The compositions set forth hereinafter show the raw duces the speci?c resistivity of the glass. batch analysis of various vanadium phosphate glasses which have been used to illustrate the effect of treating such compositions by the novel method according to the This application is a continuation of Ser. No. 265,636, 25 present invention. As shown in the examples, the ceramic ?led Mar. 18, 1963, now abandoned. semiconductors can be prepared from batch ingredients This invention relates to a method of changing the con containing a major portion (by weight) of vanadium ductivity of ceramic materials and more particularly to a pentoxide with a minor portion of numerous metal phos method of treating vanadium phosphate materials in phates. Glass compositions containing vanadium pentoxide order to change the electrical characteristics thereof. 30 in the amount of 60 to 84 weight percent in the batch The increased interest in ceramic semi-conductors has have been shown to undergo devitri?cation during reheat led to several studies of the electrical properties of vari ing. After casting the molten glass, the ceramic material ous ceramic compositions. Such studies have indicated is heated in the temperature range between the transition that glass compositions which contain high percentages of temperature and the liquidus temperature for a period of vanadium oxide produce glasses which have characteristic time sufficient to alter the resistivity. The semiconductors low resistivities. Glasses of this type have been disclosed in the British Patents 744,205 and 744,947 which were published on Feb. 1, 1956, and Feb. 15, 1956, respectively. Attempts to utilize vanadium compositions as semi conductor materials led to the development of certain 40 metaphosphate compositions which are disclosed and claimed in our copending application entitled, “Ceramic are ordinarily used at ambient temperatures. COMPOSITION A Ingredient and which nevertheless had the desired electrical proper 45 ties. It has now been found that vanadium phosphate glass containing a major portion of vanadium pentoxide can be treated according to the novel method disclosed herein in order to produce various degrees of crystallization. This crystallization is effective to produce a relatively large change in the speci?c resistivities‘of the materials. For example, a vanadium glass containing potassium meta phosphate was treated by a method according to the 55 Speci?c Resistivity as. 22 75. o NaPOs ___________________________ ._ 30. 7s 25. 0 626K COMPOSITION B v20, ______________________________ .. 71. 90 84.0 LiPO3 ____________________________ _. 28.10 10.0 } 10K } 157K } 239K } 186K } 39K } 27K } 10K } 870K COMPOSITION o v20. ............................. .. 70. 42 70. 0 PbPOa ___________________________ _. 29.58 30.0 COMPOSITION D V205 _____________________________ .1 60. 52 75. 0 KPO; ____________________________ .. 33.48 25.0 COMPOSITION E V205 _____________________________ _- 72.0 76.0 Enrol), ......................... .. 28.0 24.0 present invention to thereby reduce its speci?c resistivity COMPOSITION F from about 103 ohms per centimeter to 10 ohms per centimeter. Brie?y, the present invention contemplates heat treat ing vitreous samples to produce varying amounts of crys tallization to thereby produce a relatively large change in 60 the speci?c resistivity. This change in electrical resistance is particularly dependent upon the thermal history below the liquidus temperature but above the transformation range of the material. The aforementioned thermal history Wgt., Percent V205 ............................. . . Compositions,” Ser. No. 266,062 ?led Mar. 18, 1963, now Patent No. 3,278,317. This approach resulted in obtaining glass-es which could be produced in relatively large melts Mole Percent V705 ________________________________________ .. 65.0 Gd(PO3)2 .................................... ._ 35.0 COMPOSITION G V205 _________________ .. 70.0 Cd(PO3)2 ............. __ 30.0 COMPOSITION H V205 ________________________________________ .. 75.0 Cd(PO3)2 .................................... .. relates to a heat treaatment which may be subsequent to 65 COMPOSITION r 25.0 the normal casting and annealing procedures. The invention will now be described in more detail in connection with the accompanying drawings in which: FIG. 1 is a graphical representation showing the change 70 in resistivity with respect to the change in vanadium oxide; FIG. 2 is a graphical representation showing the rela V205 ________________________________________ ._ 60 VzO3Z3P2O5 __________________________________ ._ 40 COMPOSITION J V205 ________ .. 70 3,393,060 3 ' 4 The speci?c resistivities shown were measured prior to subjecting the samples to the novel method which is the subject of the present claims. The relationship between speci?c resistivity and meas uring temperature is illustrated by the graphical repre sentation shown in FIG. 2. The graphical data was ob COMPOSITION K V205 _________________ __ V20323P205 ___________ __ The speci?c resistiivties shown above relate to the spe ci?c resisitivites of the vitreous glasses prior to subjecting the glasses to crystallization. The glasses set forth in the various examples may be prepared in various ways. For example, the ingredients taken from the melt identi?ed as Composition D, i.e. a in an electric resistance furnace and the ingredients are melted therein at a temperature of approximately 900° C. reheated material. FIG. 3 shows the eifect of subjecting the glass taken tained by measuring the speci?c resistivity of glass samples composition containing 75 weight percent V205 and 25 in the powdered form are mixed and placed in a platinum 10 weight percent KPO3 at various temperatures. The lower curve shows the temperature-resistivity relation for the crucible. The crucible containing the mixture is placed Relatively small melt-s were made in this manner and were from the same sample identi?ed as Composition D to Larger melts were also made at this same temperature, shows that as a general rule there is a sharp reduction of resistivity as the temperatures are increased above the transition range. The rate of change decreases as the tem held at this temperature for approximately 3-4 hours. 15 progressively higher temperatures for ?ve hours. This however, were stirred according to conventional glass making techniques for approximately 4 hours. The smaller perature approaches the softening range. melts were cast at approximately 900° C. on a plate having The eifect of holding the sample 75 weight percent a temperature of approximately 100° C. In the case of 20 V205, 25 weight percent KPO3 i.e. Composition D at the the larger melts the melt was cooled to approximately same temperature i.e. a temperature which is above the 700° C, with continued stirring prior to casting on a plate transformation range, for various periods of time is shown by the graphical representation in FIG. 4. This shows that the greatest drop in resistivity occurs during the ?rst of about 100° C. All types of the glass disclosed herein were annealed at approximately 250° C. Table I illustrates the effect of heat treating vanadium half hour of treatment and appears to increase slightly when held at a longer period of time. In the case of the phosphate materials at various temperatures and for vari ous time intervals in accordance with the novel method cadmium and lithium compounds the crystallized samples disclosed herein. gave higher resistances than their vitreous counterparts. In TABLE I Temperature, 30 other cases the resistances decrease as crystallization Time occurs. Speci?c ° C. Resistivity The infra-red radiation transmission of the phosphate materials is generally similar to typical phosphate glasses. 380 0"... E ____________ _. 5 hrs _________ __ It has been noted however, as the vanadium content of 59K 260 250 706K 294K 250 276K 5K 250 250 250 260 310 290 250 270 290 310 330 350 43K 233K 5K 0. 021K 10K 5.2K 0. 65K 0v 44K [124K 0. 097K 0. 011K 290 0. 26K 290 290 290 290 310 0.12K 0.13K 0. 15K 0. 23K 240K 310 310 210K 150K these glasses is increased visible radiation is absorbed and with a further increase in vanadium content this absorp tion extends into the near infrared region of the spectrum. The transmission characteristics of the glasses are shown by the graphical representations in FIG. 5. 40 What is claimed is: 1. A method for manufacturing a ceramic semi-con ductor material comprising heating a vanadium glass in the temperature range of about 250° C. to 380° C. for at least one-half hour 45 to produce crystallization and change the resistivity of the ceramic material, said vanadium glass consisting essentially of 60 to 84 weight percent V205 and 16 to 40 percent of a metal phosphate selected from the group consisting of 50 FIG. 1 is a typical curve showing the change in re sistivity with respect to a corresponding change in com position. This curve was plotted for changes in the rates barium metaphosphate, lead metaphosphate, lithium metaphosphate, sodium metaphosphate, cadmium metaphosphate, potassium metaphosphate and vana dium metaphosphate. 2. The method of claim 1 wherein the vanadium glass of V205 in weight percent with respect to changes in Ba(PO3)2 in weight persent. These changes are shown 55 consists essentially of about 75 weight percent vanadium pentoxide and 25 percent potassium metaphosphate, and in tabular form in Table 11. wherein the glass is crystallized at about 310° C. TABLE II Ingredient xlgiai isgeli t’ References Cited UNITED STATES PATENTS Iigiilsig?y, 3,278,317 é } 1, 700K as 58: 8 5313 33:8 } } } } 193K 240K 93K 19K 65 10/1966 Blair et al. ________ __ 106—47 OTHER REFERENCES Snell: “Electrical Properties and Uses of Glass,” Glass Industry, September 1962, page 484 only. DONALL H. SYLVESTER, Primary Examiner. G. R. MYERS, R. L. LINDSAY, Assistant Examiners. UNITED STATES PATENT OFFICE CERTIFICATE OF CORRECTION Patent N0 . 3 ,393,()60 July 16 , 1968 Gerald E. Blair et a1. It is certified that error appears in the above identified patent and that said Letters Patent are hereby corrected as shown below: 47 , Column _4, line 45, "change" should read -— reduce —- ; line line 48, "40" should read -— 30 “60“ should read -- 7O — ~ ; weight -—; lines 51 and 52, cancel "cadmium metaphosphate —-. Signed and sealed this 24th day of February 1970. (SEAL) Attest: Edward M. Fletcher, Jr. Attesting Officer WILLIAM E. SCHUYLER, JR. Commissioner of Patents
© Copyright 2025