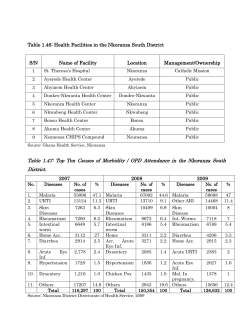
A HOSPITAL BASED STUDY OF MALARIA IN NDIEGORO BY
A HOSPITAL BASED STUDY OF MALARIA IN NDIEGORO COMMUNITY, ABA SOUTH L.G.A. ABIA STATE. BY UMEH, JUDE MMADUKA REG. NO.M.Sc/2007586009P DEPARTMENT OF PARASITOLOGY AND ENTOMOLOGY NNAMDIAZIKIWEUNIVERSITY, AWKA ANAMBRA STATE – NIGERIA SUPERVISOR: PROF.(MRS.)C.I.ENEANYA MAY-AUGUST,2009 2 TITLE PAGE A HOSPITAL BASED STUDY OF MALARIA IN NDIEGORO COMMUNITY, ABA SOUTH L.G.A., ABIA STATE. A RESEARCH PROJECT PRESENTED TO THE DEPT. OF PARASITOLOGY AND ENTOMOLOGY, NNAMDIAZIKIWEUNIVERSITY, AWKA, ANAMBRA STATE, NIGERIA BY UMEH, JUDE MMADUKA REG. NO.M.Sc/2007586009P IN PARTIAL FULFILMENT OF THE REQUIREMENTS FOR THE AWARD OF MASTER OF SCIENCE (M.Sc.) IN PARASITOLOGY AND ENTOMOLOGY SUPERVISOR: PROF. (MRS.) C. I. ENEANYA MAY-AUGUST, 2009 3 CERTIFICATION This is to certify that this research project work was done by Umeh, Jude Mmaduka. in the department of Parasitology and Entomology, NnamdiAzikiweUniversity, Awka, Anambra State, Nigeria. Sign_____________________ Prof. (Mrs.) C. I. Eneanya (Supervisor) Sign___________________ Dr. N.A. Ozumba (Head of Dept.) 4 DEDICATION This work is dedicated to God, the Almighty, for granting me good health throughout the period of my studies in this University. 5 ACKNOWLEDGEMENT I am immensely grateful to my project supervisor, Prof. (Mrs.) C.I. Eneanya for her motherly advice, constructive criticisms and painstaking efforts for the realization of this work. Special thanks go to the management and staff of Victory Christian South L.G.A. immense effort Hospital of for support located in Permission of the Mr. C. Utazi Ndiegoro to use laboratories of the Community, Aba the hospital and technologists. The Statistics Department, NnamdiAzikiwe University, Awka is highly appreciated for assisting me in the use of the Graph pad Instat Demo software package for the analysis of my results. Finally I remain ever grateful to my entire family for their co-operation and concern. 6 TABLE OF CONTENT Title page .. .. .. .. .. .. .. ii Certification .. .. .. .. .. .. .. iii Dedication .. .. .. .. .. .. .. iv Acknowledgement .. .. .. .. .. .. v Table of content .. .. .. .. .. .. vi List of tables .. .. .. .. .. .. .. vii List of figures .. .. .. .. .. .. .. viii Abstract .. .. .. .. .. .. .. ix .. .. .. .. .. .. .. 1 1.2Aims and objectives .. .. .. .. .. .3 .. .. .. .. .. 4 .. 4 .. Chapter One Introduction Chapter Two Literature Review .. 2.1 Geographical distribution of malaria .. 2.2 Epidemiology of malaria . 2.2. Environmental factors . . 5 5 2.2.2 Vectorial factors 6 2.2.3 Host factors 6 2.3 Studies on the prevalences of malaria 7 2.4 Transmission of malaria 12 2.5 Life cycle of malaria parasite 13 2.6 Pathogenesis and Pathology of malaria 16 2.7 Diagnosis of malaria 16 7 Chapter Three 3.0 Materials and Method .. .. .. .. .. 3.1 Study areas .. .. .. .. .. .. 3.2 Study subject .. .. .. .. .. 3.3 procedure .. .. .. .. .. 3.4 Qualitative data collection .. .. 3.5 Analysis of results .. .. .. .. .. . 21 21 22 .. .. .. .. 24 .. 24 Chapter Four Results .. .. .. .. .. .. 25 .. .. .. .. .. .. .. 30 Summary and Recommendations .. .. .. 34 Chapter Five Discussion Chapter Six 6.1 21 References .. .. .. .. .. .. .. 38 Questionnaire .. .. .. .. .. .. .. 52 Appendices .. .. .. .. .. .. .. 54 8 LIST OF TABLES Table 1: Age prevalence of malaria - - - - - 25 Table 2: Sex prevalence of malaria - - - - - 25 Table 3: Help-seeking behaviour of the respondents - - 28 Table 4: Preventive measures adopted - - - 28 Table 5: Methods of malaria treatments used - - - 28 Table 6: Educational background and method of treatment - 29 LIST OF FIGURES Figure 1: Geographic Distribution of Malaria - - - 4 Figure2: The Life Cycle of Malaria - - - 19 - - 9 ABSTRACT The study surveyed the prevalence of malaria as well as management practices adpted in Ndiegoro community, Aba South L.G.A., Abia state between May and August 2009. Blood samples of 300 individuals who attended local hospital were examined using Giemsa stained thick and thin films, One hundred and fifty two (152) persons (51%) were infected with Plasmodiumfalciparum . The age group 0-5 years ( 74.3%) had the highest prevalence, while the age group 36-45 years ( 40.0%) recorded the lowest prevalence in the study. Males ( 58.7%) were statistically more infected than females ( 43.3%) ( p< 0.05 ). Structured questionnaire were also administered to obtain their management practices. On the help-seeking behavior of the respondents more persons (63.4%) in the age group 0-15 years attend laboratories for diagnosis more often than those in the age group 16> (36.6%). More of the respondents resorted to patent chemist (27.6%) for treatment purposes. Visit to hospitals for treatment was the least patronized (12.0%). Some of the respondents combined more than one methods in their treatment for malaria. The use of prophylactic drugs( 6.0%) and insecticide treated nets( 1.8%) were the least preventive measures adopted by the respondents. This was due to the financial constaint and non-awareness of them. Malaria still remained a public health problem in Nigeria and data on its precise prevalence in some communities has remained unidentified. CHAPTER 1 MALARIA INTRODUCTION: Malaria is a life-threatening disease of man caused by parasite of the genus Plasmodium, which is transmitted from person to person, through the bite of infected female Anopheles mosquitoes. It is a killer and debilitating disease and remains a formidable health and socio-economic problem in the world (Nebeet al, 10 2002). Jaine and Michael (1990) described it as the leading cause of death in the developing world. The World Health Report (2002) reported that about 90% of all malaria deaths in the world today occur in Africa, South of the Sahara. And that this is because majority of the infections in Africa are caused by Plasmodium falciparum, the most dangerous of the four human malaria parasites. Anopheles gambiae is the most effective malaria vector, the most widespread in Africa and the most difficult to control. Global estimate on morbidity and mortality resulting from malaria shows between 300-500 million clinical cases and between 1.5-2.7 million deaths attributed to malaria annually (Obi, 1997; Salako, 1997; WHO, 1998 and UNICEF, 2000), and an estimated one million people in Africa die from malaria each year and most of these are children under 5 years old and women in their first pregnancy (WHO, 2002; Sherman, 1998). NIH (2001) reported that the number of deaths from malaria are on the increase due to insecticide resistance, antimalarial resistance and environmental changes. The four important species of the parasite that cause this disease are Plasmodium falciparum.P. malariae, P ovaleand P. vivax. Various species of the malaria parasites such as P.falciparum and P. malariaeare reported in Nigeria (Eneanya, 1998; Matur, et al, 2001). Anopheles gambiae, An. funestus and An. arabiensis have been implicated for malaria transmission in Nigeria with major impacts (Umaru et al, 1997). Scientific investigations revealed many pathological effects of malaria on man which include varying degrees of anaemia, splenic enlargement and various syndromes resulting from physiological and pathological involvements of certain organs like the brain, liver and the kidneys (Adams and Macgraith, 1985). Chukwuraet al (2003) described P. falciparummalaria as the 11 most prevalent and virulent in Nigeria, capable of causing mental apathy, weakness and generally slowing down economic development; accounting for up to 98% of severe cases with significant mortality and morbidity (WHO, 2000). Malaria has been observed to keep people away from school or work thereby affecting; (i) The amount they learn at school (ii) The quantity of food they are able to grow and (iii) The money they can earn (WHO, 1991). Salako (1996) and Cooker et al, (2001) reported that malaria accounts for over (600) six hundred deaths daily in Nigeria, especially in children less than five years of age in the rural, peri-urban and urban settlements; with high index of child mortality from the disease. Mbanugo and Ejims (2000) also reported that malaria is holoendemic in many countries and directly responsible for up to 10-25% of the infant mortality. Poor knowledge, attitude and practice (KAP) by our people in handling malaria seems to compound the issue of this disease in our various communities, particularly in AbiaState. Studies in Nsukka, Enugu state by Briegeret al (1997) and in a coastal area of Lagos state by Nebeet al (2002) confirmed that the perception of malaria by the inhabitants were not helpful. Many believe that malaria is caused by such factors as excessive heat, malnutrition, eating too much palm oil and other superstitious considerations. This poor malaria perception stimulated the present study ‘A study to determine the prevalence of malaria infection among members of Ndiegoro community, Aba South L.G.A., Abia State., attending hospital and to ascertain their management practices’. The outcome of the study is hopefully 12 expected to disclose some strategies for eliminating or reducing to the barest minimum this health problem of man and enhancement of his health, generally. 1.2 AIMS AND OBJECTIVES OF THE STUDY The aims and objectives of this study are; To determine the prevalence of malaria in the study area andto document the management practices by the people in the community. (ii) Specific Objectives: These are: To determine the prevalence of malaria with regards to age and sex To identify the Plasmodium species prevalent in the study area To document the help-seeking behavior of the people To document the preventive measures adopted by the people To document the treatment methods by the people 13 CHAPTER TWO LITERATURE REVIEW 2.1 GEOGRAPHICAL DISTRIBUTION P. falciparum is found mainly in the hotter and more humid regions of the world. It is the main species found in tropical and subtropical Africa and parts of Central America and South America, Bangladesh, Pakistan, Afghanistan, Nepal, Sri Lanka, Southeast Asia, Indonesia, Philippines, Haiti, Solomon Islands, Papua New Guinea and many islands in Melanesia. It also occurs in parts of India, the Eastern Mediterranean and Middle East.Plasmodium falciparum is the most commonly encountered species in West Africa including Nigeria (Seboxa and Snow,1997; Mbanugo and Ejims,2000; Aribodoret al; Asianyaet al,1999) P. vivaxis capable of developing in mosquitoes at lower temperatures than P. falciparum, and therefore has a wider distribution in temperate and subtropical areas. P. vivax is the main Plasmodium species in South America, Mexico, the Middle East, India, Pakistan, Sri Lanka, Papua New Guinea and Solomon Islands. It is also found in parts of Southern Asia, Indonesia, Philippines, Madagascar, tropical and subtropical Africa, china and Korea. P.malariae has a much lower prevalence than P. falciparumand P. vivax. It accounts for up to 25% Plasmodium infection in tropical Africa. It is also found in India, Guyana, Malaysia and Sir Lanka. In these countries, it accounts for less than 10% of Plasmodium infections. Plasmodium ovale has a restricted distribution and of low prevalence. It is found mainly in West Africa where it accounts for up to 10% of malaria infections. It has also been reported from other parts of the Far East, South East Asia and South America. 14 2.2 EPIDEMIOLOGY OF MALARIA The setbacks in attempts at malaria eradication have led to renewed interests in the epidemiology of malaria. Various factors interplay to determine the two epidemiological extremes of malaria, that is, stable and unstable malaria. Such factors include environmental (or climatic), vectorial, parasite and host factors and failure of control policies. Malaria not only increase malaria- specific morbidity and mortality but also affect the general health of the population. Many malaria epidemics coincide with periods of famine, economic meltdown, war or civil disturbances involving impoverished populations that are often affected by other disease as well. The greatest consequences of malaria cases in many parts of the world stems in large part to these economic and human factors (Dick, 1985). 2.2.1 Environmental factors The potential of the mosquito to serve as a vector depends on the ability to support sporogony, mosquito abundance, and contact with humans, which are all influenced by climatic and ecological factor. Temperature and mosquito longevity are other key factors affecting the parasite’s interaction with the vector. Development of P. falciparum requires a minimum temperature of 200C, whereas the minimum temperature for the other species is 16 0C. Temperature also affects the time of development in that the duration of sporogony is shorter at higher temperatures. A shorter duration of sporogony increases the chances that the mosquito will transmit the infection within its lifespan .Climatic factors such as; altitude, temperature and rainfall and breeding places also affect mosquito density. Small increases in existing low temperatures have been shown to exert a strong effect on increased transmission of malaria (Bradley, 1993; Lindsay and Birley, 1996).In a site situated at 2000m in western Kenya, malaria cases steeped when mean monthly temperature exceeds 18C and rainfall greater than 1500mm per month(Malakootiet al, 1998).Wet and humid environments provide the breeding 15 sites and prolong the life of malaria vectors (Lindsay and Birley, 1996). These optimum conditions occur through out the year in most part of Nigeria and hence the prevalence rate is fairly constant throughout the year in both urban and rural areas. At high altitude, prevalence rate of malaria is low and no transmission occurs at 600 feet above sea level(Taylor and Mutambu, 1986). 2.2.2Vectorial Factors: The Mosquito (Anopheles spp.) These are behavioral factors and susceptibility to infection. Some species of Anopheles are anthropophilic (prefers human blood), others zoophilic (prefers animal blood); some prefer to bite indoors (endophagy), others outdoors (exophagy); some prefer to rest during the day indoor(endophily), others out doors (exophily). Malaria vectors bite between dusk and dawn and generally choose well-oxygenated water rather than stagnant polluted pools to lay her eggs. Anopheles gambiae, Anopheles arabiensis and Anopheles funestustransmit most of human malaria and are all found in Africa (Besanskyet al, 2004). An. gambiae, the most famous and significant of the three, is one of sixty anopheline mosquitoes able to transmit malaria to humans (Budiansky, 2002). An gambiaeis the primary malaria vector, this can be attributed, in part, to its relatively long life strong anthropophily and endophily.Their larvae tend to develop in temporary water bodies, such as those typically found near agricultural sites or even in flooded hoof print (Vogel, 2002). All these characteristics combine to make An. gambiaethe mostsuccessful vector. 2.2.3 Host Factors: The following human factors influence the epidemiology of malaria: genetic, immune, nutritional and behavioral. Behavioural Factors: 16 Many elements of human behavior profoundly affect the epidemiology of malariauncontrolled urbanization; subsistence agriculture; population movements; woodgathering in the forest; open-cast mining; gem-silver and other mining, agricultural production of cotton, sugar-cane, rubber and rice. Behavioral patterns emerge in different communities and are influenced by cultural, ethnic and religious backgrounds. The introduction of electricity into rural areas has resulted in promoting late-night outdoor activities and thus increased biting opportunities for mosquitoes. Genetic factor Some inherited disorders of haemoglobin such as sickle cell confer a reasonable degree of resistance or immunity against malaria to certain groups of individuals. It has been found that those individuals who are heterozygous for haemoglobin (AS) suffer malaria less frequently and less severely than to normal individuals (Olumeseet al, 1997). It has also been found that those individuals with ß – thalassaemias are protected against malaria. In a clinic-based case control study in Northern Liberia, sickle cell carries showed about 70% reduction in their risk of clinical malaria while carries of ß –thalassaemias showed 50% reduction in risk (Willcoxet al ;1983). 2.3 STUDIES ON THE PREVALENCE OF MALARIA High prevalence of malaria has been reported in various parts of Nigeria. In a Survey of the prevalence of Plasmodium species and common clinical symptoms in a rural community in ImoState, Chukwuochaet al, (2008) found a 68% prevalence of malaria in the study community. They reported that P.falciparum(67.8%) was the dominant parasite while P.malariae occurred in mixed infection with both parasites at a 0.9%. 17 Ukpai and Ajoku (2001) in a hospital-based study of the prevalence of malaria in Okigwe and Owerri areas of ImoState, reported a prevalence of 321(80.25%) out of 400 individuals examined. Okigwe had a higher prevalence rate (85.5%) than Owerri (75.00%). Infections with P. falciparum was the highest in the two areas studied with 53.00% infection rate at Owerri an 60.00% infection rate at Okigwe. Some authors have reported a significantly higher prevalence in males than females (Obiukwu and Okwuon (2008), Ikeh and Udem (2008), Ukpai and Ajoku (2001); Matur et al, (2001). Studies on prevalence of malaria and management practices of the Azia Community in Ihiala L.G. A., AnambraState, South east, Nigeria, showed a 76% prevalence rate of malaria, all were infections of Plasmodium falciparum. (Aribodoret al, 2004). Matur et al (2001) in a study of prevalence of malaria parasites amongst the undergraduates of the university of Abuja reported a prevalence rate of 121 (61%) out of 200 blood samples examined.Similarly, Mbanugo and Ejims (2000) reported a prevalence of 233 (58.3%) out of 400 children examined for malaria parasites in Awka Metropolis, AnambraState. All positive cases were infections of Plasmodim falciparum. A three year study to investigate the seasonal variations in episodes of malaria among residents in a semi-urban community in south east Nigeria, showed that between January and December 1996, 755 (62.9%) individual had parasitaemia of either P. falciparum or P. malariaeor both. The age-specficprevalences were: 73.8% for (0-4) years; 76.4% for (5-9) years, 67.2% for (50-59) years; 43.5% for 60 years (Eneanya, 1998.)Usip and Opara (2008), reported a malaria prevalence of 552 (54.4%) out of 1, 012 patients attending St. Luke’s General Hospital, Anua, Uyo State between May 2003 and April 2004. All malaria positive cases reported were due to Plasmodium falciparum. 18 In a study on the prevailing knowledge, attitude and practices (KAP) amongst mothers and caregivers in Aba South L.G.A., AbiaState towards malaria infection, Ukpai and Amaechi (2008) reported a remarkable mix-up of traditional and modern medications (orthodox) among the respondents in the treatment of malaria. A good number of he respondents (53.19%) visited the chemist each time they fell ill of malaria. Some (37.85%) used herbs called ‘OgwuIgbo’.Some of the herbs were boiled before drinking, inhaled or used to bathe. Only a few of the respondents(5.98%) visited the hospital. Prevention against mosquito bites included use of aerosols (17.13%), use of mosquito coils (49.60%), and use of nchawu (15.74%) among others. Aribodoret al (2004), reported that the management practices of the Azia community, IhialaL.G.A.AnambraState include: attend hospitals (24.6%); use traditional medicine from local healers (12.0%) and buy anti-malarial without physician prescription (25.0%). Similarly result from Ibeju-Lekki communities of South Western Nigeria (Nebeet al, 2002) reported a general low level of knowledge on the management practice adopted by mother and care providers. The prevailing methods of management of childhood convulsion were noted: traditional healers (26.4%), health centers (16.6%), among others. Furthermore, Obiukwu and Okwuonu (2008) in a study of prevalence of malaria and management practices adopted in Abba Njikoka L.G.A., Anambra State, Nigeria reported that greater number of respondents resorted to patent medicine 128 (36.6%) for treatment purposes. None of the poorest respondents used prophylactic drugs and insecticide treated nets. They attributed this to financial constriant and non-awareness of these preventive measures by the people. 19 Oyewole and Ibidapo (2007) in a study of attitudes to malaria prevention, treatment and management strategies associated with the prevalence of malaria in a Nigerian urban center, reported that preventive measures adopted against mosquito bite include sleeping under net (treated and untreated) 17(4.2%), door and window screening 37(9.2%),cover cloth 55(13.8%), mosquito repellant/insecticides spray 39(9.8%), environmental hygiene 26(6.5%), herbal decoction 26(6.5%), and chemoprophylaxis 45(11.3%). Also in the study, self treatment (medication) accounted for 267(66.8%) as against hospital treatment 93(23.3%).Attapeu provincial Health service (2003) in a survey of knowledge, attitude and practice (KAP) in Lao PDR reported that 48.5% responded that they visit a doctor for treatment in hospital, 17.8% said they go to a health center, 51.8% goes to buy and take medicine, by themselves and 9.5% undergoes traditional healing practice. The preventive strategies adopted include: sleeping under mosquito nets (91.3%), drinking boiled water (15.5%), keeping the house and surrounding clean (54.8%) and wearing long sleeves shirts (23%). Legesseet al(2007) in a survey of KAP about malaria transmission and its preventive measures among households in urban areas of Assosa zone, western Ethiopia, reported that the major malaria preventive measures includes: sleeping under a mosquito net (55%) and eliminating mosquito breeding sites (52%) respectively. Abd/El-Gayoumet al (2006) in a survey of knowledge, practices and perceptions which affect acquiring malaria in man –made malaria area in Khartoum state, Sudan reported that the preventive measures against malaria were: insecticides use (22.9%),bed nets (23.9%), screened window (25.8%) and 39.5% reported no attempt to use any preventive measures. Among the treatment methods adopted were: health centers (83.2%), private clinics (65.9%) and hospitals (42.5%) 20 Commenting on malaria control, through treatments, Foster (1995) while investigating on rural malaria in Gambia, reported that a large proportion of malaria patients in most endemic areas, receive some form of treatment in the home or community without ever making contact with the formal health services, and that home treatment of fever in Africa South of the Sahara can account for up to 75% of all cases. Ezedinachiet al (1997) working on perception of malaria infection by people in two rural communities (Awi and IkotEdemOdo) in Cross River State of Nigeria, also reported that unspecified drugs and traditional medicines were initial treatment responses, while formal health sector was consulted only if home initiated measures failed. Also Uzochukwuet al (2008) while investigating Rural-Urban (AmechiAwkunanaw – Uwani) differences in maternal responses to childhood fever in South East, Nigeria, reported that surveys in Africa revealed that 80-90% of fever presumed to be malaria cases were treated at home, while formal health care is sought only if initial treatment fails. Tore (1995) reported, in WHO 12thProgramme Report, (1995) that in most malaria treatment, 80% in malaria endemic areas like Nigeria is handled at home without microsopic confirmation of the suspected malaria episode. Coker and Adesegun (2006) were also of same opinion by reporting that in Africa almost 70-80% of the population patronise traditional healers for their medical care, and that in rural communities, the herbalists or traditional healer is usually the first port of call in the event of illness. Salakoet al (2001) and Iweze (1987) reported that in Nigeria, patent medicine stores (PMS) are usually the first choice in health care and a recognised primary source of orthodox drugs fro both rural and urban populations, especially the poor. 21 2.4 TRANSMISSION OF MALARIA On transmission of this disease, Ernest et al (1974) reported that the transmission of malaria is limited to the tropics and subtropical regions of the world. However, they remarked that in the past, transmission occurred in many temperate regions and that in the zone, malaria was unstable and relatively easy to control or eradicate, while tropical malaria is often more difficult to eradicate. This is very evident in Nigeria. Markellet al (1999) reported that transmission of all species of malaria parasites depends on the presence of both the suitable species of Anopheline mosquitoes and of the (gametocyte-bearing) humans, and that the suitability of a mosquito as a vector of human malaria disease depends not only on the physiologic adaptation to the infection, but also on such factors as feeding preferences, hour of biting and flight, resting and breeding habits. They also reported that favourable breeding places abound in the tropics, such as old cans, coconut shells, wells, old tyres, poor drainage systems, bushes and hedges around dwelling houses etc. These factors, obviously, promote mosquito multiplication. Apart from transmission through the bites of infected female Anopheles mosquitoes, transmission through blood transfusion and mechanical transmissions through shared syringes by drug users are also known. The World Health Report (2002) remarked that in areas of stable malaria transmission, very young children and pregnant women are the population group at highest risk for malaria morbidity and mortality, and that most children experience their first malaria infection during their first year or two of lives, when they have not yet acquired adequate clinical immunity – which makes these early years particularly dangerous. Commenting 22 also on transmission, Murphy and Breman (2001) reporting on malaria transmission in urban sub-Sahara Africa stated that a review of malaria transmission in sub-Sahara Africa cities shows the strong likelihood of transmission occurring within these sprawling cities, whatever the size or characteristics of their bioecologic environment. However, considerable variation in the level of transmission exists among cities and within different districts in the same city. 2.5 Life Cycles of Malaria Parasites Plasmodium undergoes three (3) cycles: two (2) asexual cycles, which occur in man and the sexual cycle, which occurs in the mosquito vector Liver stage: Human infections are initiated when sporozoites are injected with the saliva during the infected female Anopheles mosquito feeding. The sporozoites enter the circulatory system and within 30-60 minutes will invade a liver cell. The speed and selectivity of the process have indicated that sporozoite invasion of heptatocytes involves parasite-encoded surface proteins and host molecule(s). Despite the body of information that is available on the biology of the sporozoite, and the hepatic stages of Plasmodium, the exact route of sporozoites to their target cells is still not entirely clear (MeisansVerhave, 1998; Sinnis and Sim, 1997). After invading the heptocytes, the parasite undergoes an asexual replication. This replicative stage is often called exoerythrocytic (or pre-erythrocytic) schizogony. Schizogony refers to a replicate process in which the parasite (schizonts) undergoes multiple rounds of nuclear division without cytoplasmic division followed by a segmentation, to form a progeny. The progeny, called merozoites are released into the circulatory system following rupture of the host hepatocyte. In P.vivax and P.ovale, some of the sporozoite do not immediately undergo a sexual replication, but enter a dormant phase known as the hypnozoite. This 23 hypnozoites can reactivate and undergo schizogony at a later time resulting in a relapse. Relapse refers to the reactivation of the infection via hypnozoites. Recrudescence is used to describe the situation in which parasitaemia falls below detectable levels and then later increases to a patent parasitaemia, as it is found in Plasmodium falciparum and P.malariae Blood Stage: Sometimes called the erythrocytic stage is initiated with the release of approximately 105 to 106merozoites from the matured and ruptured schizonts in the liver. This is the product of 5 to 100 successful sporozoites. These merozoites liberated into the blood circulation invade passing red blood cells immediately. After entering the erythrocyte the parasite undergoes a trophic period followed by asexual replication. The young trophozoite is often called a ring form due to its morphology in Giemsa-stained blood smears. As the parasite increases in size, this ‘ring’ morphology disappears and it is called a trophozoite. Nuclear division marks the end of the trophozoite stage and the beginning of the schizont stage. Erythrocyticschizogony consists of 3-5 rounds (depending on species) of nuclear replication followed by a budding process. Late stage schizonts in which the individual merozoites become discernable are called segmenters. The host erythrocyte ruptures and releases the merozoites. These merozoites invade new erythrocytes and initiate another round of schizogony. The blood stage parasites within a host usually undergo a synchronous schizogony. The simultaneous rupture of the infected erythrocytes and the concomitant release of antigens and waste products accounts for the intermittent fever paroxysms associated with malaria. 24 Sexual Stage: As an alternative to schizogony, some of parasites will undergo a sexual cycle and terminally differentiate into either micro- or macro gametocytes. The factors involved in the induction of gametogenesis are not known. However, commitment to the sexual stage occurs during the asexual erythrocytic cycle that immediately precedes gametocyte formations. Daughter merozoites from thisschizonts will develop into either all asexual forms or all sexual forms. Gametocytes do not cause pathology in the human host and will disappear from the circulation if not taken up by a mosquito. Gametogenesis is inductive when the gametes (micro and macro) are ingested by a mosquito. After ingestion by the mosquito, the microgametocyte undergoes three rounds of nuclear replications. These eight nuclei then become associated with flagella that emerge from the body of the microgametocyte. This process is readily observable by light microscopy due to the thrashing flagella and is called exflagellation. The microgametocyte mature into microgametes. Ex-flagellation occurs spontaneously when infected blood is exposed to air. Critical factors involved in the induction of this gametogenesis include: decrease in temperature, a decrease in dissolved CO2 and the subsequent increase in PH to above 8.0. The highly mobile microgamete will seek out and fuse with a macrogamete within 12-hours, the resulting zygote develops into an Ookinete. The Ookinete is a motile invasive stage, which will traverse both the peritrophic matrix and the midgut epithelium of the mosquito. Sporogony: After reaching the extracellular space between the epithelial cells and the basal lamina, the Ookinete develops into an Oocyst. The Oocysts undergoes an asexual replication called sporogony, this generally takes 10-28 days depending on species and temperature. Upon maturation, the Oocyst, ruptures and release the 25 sporozoites which migrate to the salivary gland of the mosquito (Good et al, 2001). Some of these sporozoites will be expelled into the vertebrate host as the mosquito takes a blood meal, and thus reinitiate the infection in the vertebrate host. Malaria can also be transmitted through blood transfusion from infected person or transplacentaly from pregnant mother to the fetus. However, transmission of this nature accounts for a negligible percentage. 2.6 Pathogenesis and Pathology of Malaria Most of the major clinical manifestations of malaria may be attributed to two general factors: (1) the host inflammatory response, which produces the characteristic chills and fever as well as other related phenomena and (2) Anemia, arising from the enormous destruction red blood cells. Severity of the disease is correlated with the species producing it. Falciparum malaria is most dangerous and quartan (P. malariae) and ovale (P. ovale) are the least dangerous. Typical malaria fever attack: Fever is a common, nonspecific reaction of the body to infection, functioning at least in part to increase the rate of metabolic reactions important in host defenses. Malaria fever is correlated with the maturation of merozoites and rupture of the red blood cells that contain them. The malaria fever is stimulated by the waste products of the parasites, released when erythrocytes lyses. Release of these malarial toxins (hemozoin) into the circulation triggers a burst of tumor necrosis factor, or TNF from activated macrophages (Kwiatkowski, 1995). Introduction of fever is among the effects of overproduction of TNF, and TNF toxicity can account for most or all of the typical symptoms. A few days before first paroxysm, the patient may feel malaise, muscle pain, headache, loss of appetite and slight fever; or the first paroxysm may occur abruptly, without prior symptoms. A typical attack of benign tertian (P. falciparum) or quartan (P. malariae) begins with a feeling of intense cold and rise in temperature up to 104 oF to 106oF. The teeth chatter and the patient shivers. The 26 hot stage begins within one half to one hour later, with intense headache, temperature rises to maximum, back and joint pains, vomiting and diarrhea. As copious perspiration signals the end of the hot stage, the temperature drops back to normal within two to three hours, and the entire paroxysm is over within 8 to 12 hours. The time periods for the stages are usually somewhat shorter in quartan malaria, and the paroxysms recur every 72 hours. In vivax malaria the periodicity is often quotidian early in the infection. Because the synchrony in falciparum malaria is much less marked, the onset is often more gradual, and the hot stage extended. The fever may be continuous or fluctuating, but the patient does not feel well between paroxysms, as in vivax and quartan malaria. Malaria caused by P. falciparum (called subtertian or malignant tertian malaria) is the most widespread, accounting for up to 80% of all malaria cases world wide (Markell and Voge,1992). P. facilparumis the most pathogenic of the human malaria species with untreated infections causing severe disease and death, particularly in young children, pregnant women and non-immune adults. The pathogenicity of P. falciparum is mainly due to: High levels of parasitaemia resulting in the activation of cytokines and the destruction of many red cells. Up to 30-40% of red cells may become parasitized. The cytoadherence of falciparum parasitized red cells causing the cells to adhere to one another and to the walls of capillaries in the brain, heart, spleen, lung, intestine and placenta. Severe falciparum malaria is associated with: cerebral malaria, black water fever, severe anemia, hypoglycemia, algid malaria and complications in pregnancy. Cerebral Malaria: The most common complications of malaria, which may account for 10% falciparum malaria cases admitted to the hospital and 80% of such deaths (WHO, 1986). Cerebral malaria may be gradual followed by a coma, an uncontrollable rise in temperature to above 1080F, and convulsions, especially in 27 children. Death may ensue within a matter of hours. Many parasitized cells can be found in the capillaries of the brain and in the late stages, hemorrhaging from small blood vessels can occur. Anemia: Ogun (2006) described anemia as the commonest complication of malaria and the complexities is due to the destruction of both parasitized and nonparasitized erythrocytes, inability of the body to recycle the iron bound in hemozoin and an inadequate erythropoietic response of the bone marrow. This can be severe and occur rapidly, particularly in children. Why such large numbers of nonparasitized red cells are destroyed is still not understood but some evidence has indicated complement mediated, autoimmune hemolysis. Both the splenic removal of red cells and the defective bone marrow response may be due in part to TNF toxicity (Clark, 1987; Mendis and Carter, 1995). Destruction of erythrocytes leads to an increase in blood bilirubin. When excretion cannot keep up with formation of bilirubin, jaundice yellows the skin. Blackwater Fever: This is a rare but acute condition in which there is a rapid and massive intravascular haemolysis of both parasitized and non parasitized red cells resulting in haemoglobinaemia, haemoglobinuria, and fall in haemoglobin. It is fatal due to renal failure. Following a hemolytic attack the parasites are difficult to find in the blood. The urine appears dark red to brown-black (hence the name blackwater fever) due to the presence of free hemoglobin. The urine also contains protein, hyaline and granular casts, and epithelial debris. Blackwater fever can occur in non-immune adults with severe falciparum malaria, and also as complication of quinine treatment. Hypoglycemia: This is a condition associated with reduced concentration of blood glucose. It is a common finding particularly in children and in women with uncomplicated or severe malaria who are pregnant or have recently delivered as well as in other cases of severe falciparum malaria (WHO, 1986). This condition is usually associated with quinine treatment. The pancreatic islet cells are stimulated by quinine to increase insulin secretion, thus lowering blood glucose (Warren, 1987). This may also be to due to excessive TNF (Warren et al, 1986). 28 Algid Malaria: This condition result when the adrenals are involved. There is sudden fall in body temperature. It may be associated with abdominal pain, vomiting, and diarrhea. The may be generalized vascular collapse followed by shock. 2.7 DIAGNOSIS OF MALARIA Prompt and accurate diagnosis of malaria is the key to effective disease management. It is thus of concern that poor diagnosis continues to hinder effective malaria control. This is due to a combination of factors, prevalence of asymptomatic infection in certain areas, lack of resources and insufficient access to trained health care providers and health facilities and widespread practice of self-treatment for clinically suspected malaria. Several approaches to the diagnosis of malaria can be adopted; each present characteristics such as cost, ease of performance and accuracy. Clinical diagnosis is the most widely used approach. It has been the only feasible one in many situations, particularly in rural areas and at the periphery of the health care system where laboratory support to clinical diagnosis does not exist. Among the many clinical signs and symptoms associated with malaria, the most prominent is fever, which is often accompanied by chills, perspiration, anorexia, headaches, vomiting and malaise. In addition to these symptoms of uncomplicated malaria, other manifestation may develop that signal severe malaria, which is almost always due to P. falciparum. These include drowsiness with prostration, severe anemia, cerebral malaria, splenomagaly, hepatomegaly and others. Clinical diagnosis is easy to perform and require no special equipment. However, the symptoms of malaria are very non-specific and overlap with those of other febrile illnesses. A diagnosis of malaria based on clinical grounds alone is therefore unreliable and when possible should be confirmed by laboratory tests. Conventional light microscopy is the established without for the laboratory confirmation of malaria. The careful examination of an expert microscopist of a well prepared and well-stained blood film remains currently the gold standard’ for 29 detecting and identifying malarial parasites. In most cases, the procedure consists of collecting the smear and examining the smear through a microscope for the presence of malaria parasites. Microscopy offers such advantages as: a. It is informative since parasite species and the circulating stage is detected. b. It is relatively cheap and the technique can be shared with other disease control program. c. It is sensitive and can detect densities as low as 5-10 parasites, per microlitre of blood by skilled and careful technicians. d. It can provide a permanent record (the smears) of the diagnostic findings. The principal demerits of microscopy include being time-consuming requiring (at least 60 minutes), labor-intensive, depend absolutely on good techniques and delayed results. The third approach to malaria diagnosis is the rapid diagnosis tests (RTDS). These tests are based on the detection of antigens derived from malaria parasites in lysed blood, using immunochromatographic methods. Most frequently they employ a dipstick bearing monoclonal antibodies directed against the target parasite antigens. The test can be performed in about 15 minutes. RDTs are simpler to perform and to interpret(Quintaniaet al 1998). 30 CHAPTER THREE 3.0 Materials and methods 3.1 The Study Area The study area is Ndiegoro, a semi-urban community in Aba South L.G.A. in AbiaState southeastern Nigeria. It is located between latitude 8 o and 10oNof equator and longitude 8o and 10o E of the meridian. The vegetation is typically rainforest. The mean annual rainfall of about 2250 to 2500mm and mean annual temperature is 25 to 27oc with high relative humidity. The rainy season is observed from May to October while the dry season runs through the months of November to April. The community is made up of four villages namely: Umudike, Umuokorie, Umuonyinke and Umuzogwu. The occupation of the people includes: subsistence farming sometimes combined with petty trading. The main crops farmed include: cassava, vegetables, cocoyam and yams. 3.2The Study Subjects. The study subjects include inhabitants, who had resided in Ndiegoro community for one year and above. The sample population includes all those attending local hospitals.The samples collection was done at Victory Christian hospital, located in the community. Permission from the medical director was sought upon presenting to him the motive of the study. Microscopy was also carried out in the laboratory section of the hospital. Informed consent was obtained from the participants in the study. For children in the study, the consent of their parents was obtained prior to finger prick blood collection. 31 Procedure: Before the collection of the blood, question was put to the patient whether any anti-malarial drugs have been taken recently (less than past two weeks).Those who answered in the positive were dropped while blood samples were collected from those who answered in the negative. After the patient information has been recorded in the appropriate form, the blood films are made as follows. 1. With the patient’s left hand, palm upwards, the forefinger was selected . Cotton wool lightly soaked in methylated spirit was used to clean the finger. With a clean cotton wool the finger was dried, using firm strokes to stimulate blood circulation. 2. A disposable sterile blood lancet was used to puncture the ball of the finger. By applying gently pressure to the finger, the first drop of blood was expressed and wipe it away with dry cotton wool. Care was taken to ensure that no strands of cotton remain on the finger. 3. Handling clean slide only by the edges, the blood was collected as follows: Gentle pressure was applied to the finger and a single small drop of blood was collected onto the middle of the slide, this is for the thin film. Further pressure was applied to express more blood and two or three large dropswas collected on the slide about 1cm from the drop intended for the thin film. The remaining blood was wiped away from the finger with cotton wool. 4. Thin Film: Using another clean slide as a “spreader”, and with the slide with the blood drops resting on a flat, firm surface, the small drop of blood was touched with the spreader and the blood allowed to run along 32 its edge. The spreader was firmly pushed along the slide, away from the largest drops, keeping the spreader at an angle of 450. I ensured the spreader is in even contact with the surface of the slide all the time the blood is being spread. 5. Thick Film: Always handle slides by the edges, or by a corner, to make the thick film as follows: using the corner of the spreader, the larger drops of blood was quickly joined and spread to make an even thick film. Care was taken to avoid the blood to be excessively stirred. The blood could be stirred in a circular form with 3-6 movements. 6. The blood film was then allowed to air-dry with the slides in a horizontal position in a safe place. Label the dry film with a marker pencil by writing across the thicker portion of the thin film the patients’ number and date. STAINING BLOOD FILMS WITH GIEMSA STAIIN 1. The film was allows to air-dry thoroughly. 2. The thin film was fixed by gently dabbing with cotton wool dampened with methanol for 1-2 minutes. Care was taken to ensure that the methanol does not touch the thick film. 2. Gently pour the prepared stain (10% Giemsa solution) on the slide in a staining dish. 3. Stain for 5-10 minutes. 4. Gently flush the stain off the slide by adding drops of clean water; do not tip the stain and then wash, as this will leave a deposit of scum over the smear. 5. The slide was placed in the rack, film side downward, to drain and dry, making sure the film does not touch the slide rack. 33 REPORTING BLOOD FILMS FOR MALARIA PARASITES Blood films were examined microscopically using the x40 and x100 objectives. The 7 x eyepiece was used according to WHO (1991) and Payne (1993). The thick film allow for the detection of the presence Plasmodium. The film was considered positive if the ring forms trophozoite or any blood stage of erythrocyte schizogony was detected. The thick film was considered negative if no parasite were seen after scanning at least 100 fields. The thin films allows for the identification of the species of plasmodium including staining and morphological features. The plasmodium species were identified using the key according to Cheesbrough (1998). 3.4 Qualitative Data Collection The structured questionnaires were used. The section A of the questionnaire was for Bio-data such as: age, sex, marital status, educational qualifications. Questions on the help-seeking behavior pattern of the population was asked in section B. Questions on the preventive measures adopted was asked in section C. Questions on the treatment methods such as: buy anti -malarial drugs from chemist, attend hospitals, use herbs was in section D. 3.5 Analysis of Results: The quantitative data were analyzed using: tabulations, percentages, bar charts and test of statistically significant differences using chi-square (X2). The statistical package used was the Minitab software. 34 CHAPTER FOUR 4.0 RESULTS The results showed that 153 patients were positive for malaria parasite out of the 300 sampled. Therefore the prevalence of malaria was found to be 51.0% for the period between May and August, 2009. The prevalence of malaria with regards to age groups were found to be statistically significant (p<0.05)(Appendix 1), the 35 age-specific prevalence rate was also found as follows: 74.3% for age (0-5) yrs, 58.3% for (6-15) yrs, 41.7% for (16-25) yrs, 50.0% for (26-35)yrs, 40.0% (36-45) yrs, 45.0% for (46>) yrs. (Table 1). It was also observed that all the malaria cases detected were infections of only P. falciparum. No cases of mixed infections were identified. More males 88 (58.7%) were found to be infected than females 65 (43.3%) out of 150 randomly selected male and female each from the sampled population. (Table 2). The sex specific prevalence were found to be statistically significant (p<0.05)(Appendix 2). The help-seeking behavior of the respondents was summarized in table 3 On the management practices of malaria, the following preventive measures were identified by the respondents: Door/window (10.70%), insecticide bed nets (treated and untreated)(9.00%), cover cloth (11.70%), Mosquito repellant/insecticidal spray (23.00%), environmental sanitation (16.70%), using fan (21.70%) and 1.70% did nothing to prevent mosquito bite (Table 4). The treatment methods of the sampled population was found to include: Buying of anti-malarial drugs from chemist shops, attending local hospitals, use of traditional medicine (herbs), while few have no management at all. Of the 300 patients sampled, 79 (27.6%) buy anti-malarial drugs from chemist without prescription, 34 (12.0%) attend hospitals while 60 (21.1%) use traditional medicine (herbs) in their treatment of malaria. It was also found that some individuals use more than one method in the treatment of malaria. 24 (8.3%) combined the use of traditional medicine and attend hospitals, 41 (14.4%) combined the use of anti malarial and traditional medicine and 21 (7.4%) combined the use of anti-malarial and attend hospital. It was also observed that the practice of using more than one method was 36 by trial and error means and not that they are used concurrently. 12 (4.2%) of the respondents did nothing about treatment of malaria (table 4) Results on the influence of educational background in the malaria management of the people are summarized in Table 5. Of the 300 individuals sampled, 106 (37.2%) had informal education, 63(22.1%) had primary education, 73(25.6%) had secondary education and 43(15.1%) had tertiary education. It was observed that the highest percentage of those who attend hospitals to treat malaria had secondary and tertiary education while the highest percentage of those who buy anti-malarial drugs over the counter and as well use traditional medicine form healers had informal or primary education. 37 Table 1:Age Prevalence of malaria infections in the community Age groups (yrs) No. Examined No. Infected % 0-5 35 26 74.3 6-15 72 42 58.3 16-25 72 30 26-35 56 28 50.0 36-45 45 18 40.0 46> 20 8 TOTAL Df=8, 41.7 45.0 300 p=0.032, p<0.05 153 51.0 Table 2: Sex-prevalence of malaria infection in the community SEX NE Male 150 88 (58.7) Female 150 65 (43.3) Total 300 153 (51.0) Df=1, NI (%) p=0.008, p<0.05 Table 3:Help-Seeking Behaviourof Respondent Age group (yrs) Lab Diagnosed yes (%) Lab Diagnosed Occasionally (%) Lab Diagnosed No (%) Total 0-15 59 33 15 107 16 > 34 117 27 178 38 Total 93(32.6) 150(52.6) 42(14.7) 285 Table 4: Preventive Measures Adopted Agegroup Doors + window bed nets Screen (%) (%) 0-15 18 16 >14 Untreated ITNS Cover Mosquito EnvtalUsing Insecticide Chemopro- None (%) cloth repellant Sanitation fan spray phylaxis (%)(%) 15 4 7 Total 32(10.70) 1 22(7.30) (%) 25 10 5(1.70) (%)(%)(%) 9 21 31 35(11.7) Total 29 40(13.30) 20 45 50(16.7) 10 19 65(21.7) 6 1 11 29(9.7) 4 1 7(5.7) 129 171 5(1.7) 300 Table 5: Methods of malaria treatment adopted Age Buy Group antimalarial (yrs) from Chemist (%) Attend hospital (%) 0-15 32 23 12 8 12 16> 47 11 48 16 29 60(21.1) 24(8.3) 41(44) Total 79(27.6) 34(12.0) Use traditional medicine (%) Trad. Med. + Attend hosp. Antimalarial + Trad. Med. (%) Antimalarial + Attend (%) Hosp. Antimalarial None trad. Med. (%) Total 14 5 2 107 7 9 10 178 12(4.2) 285 21(7.4) 14(5.0) Table 6: Educational background and the method of treatment of the people Treatment method Respondents Informal Primary Secondary Tertiary Buy antimalarial from Chemist 79 34 16 30 9 Attend hospital 34 5 7 9 13 Use traditional Medicine 60 35 13 8 4 14 8 4 2 0 Traditional medicine and Attend Hospital Antimalarial and Traditional medicine Antimalarial, Hospital 41 20 11 7 3 39 and traditional medicine 21 10 None 120 2 Total 285 106 5 6 63 4 2 4 73 43 CHAPTER FIVE Discussion: Malaria is acknowledged to be by far the most important tropical parasitic disease causing great suffering and loss of lives (WHO, 1993).The days of labor lost, the cost of treatment of patients and the negative impact of the disease make malaria a major social economic burden (WHO, 1993). Results from the study indicate that more than half (51.0%) of the individuals examined were positive for malaria parasites in their blood.Table 1 shows the prevalence of malaria infection with regards to age groups with the age group (05) years recording highest prevalence (74.3%). The trend of this result agrees with Salako, (1994) and Steketee, (2001). Steketee, (2001) reported that in areas of stable malaria transmission, very young children are the population group at highest risk for malaria morbidity and mortality, and that most children experience their first malaria attack during their first or two of life, when they have not yet acquired adequate clinical immunity. He also reported that 90% of all deaths from malaria occur in Africa in young children. 40 High prevalence rate in the age group (0-5) could be due to low transferred maternal immunity or inadequate protection. Also the age group (6-15) and (1625) which constituted 58.30% and 41.70% of the population studied belong to the school aged class. This, therefore, may suggest that malaria is a major cause of school absenteeism among the population during the period the study was undertaken. According to WHO (1996), Malaria has been found to be one of the most common cause of school absenteeism, affecting over one –third of primary school children during a school term with more than half of the students having up to two attacks, typically missing a week more of school with each attack. Interestingly only the ring form stage of Plasmodium falciparum represents a major public health problem in Nigeria. In related studies carried out in Awka South East Nigeria (Mbanugo and Ejims, 2000), and in LagosState, South West Nigeria (Asianyaet al, 1999), and in Azia, AnambraState (Aribodoret al, 2003) only infections of P. falciparum were reported. These findings contrast with other studies (Ukpai and Ajoku, 2001; Matur et al 2001) were cases of P.faliciparumand P. malariaeor mixed infections with P. falciparum and P. malariaewere reported. It is however, pertinent to state that malaria caused by P. falciparum is the most widespread, accounting for up to 80% of malaria in Africa (WHR, 2002, Markell and Voge,1992). Sex-wise, more males were infected (58.7%) than females (43.3%) (table 2). This could be due to the fact that the male expose themselves more than females especially when the weather is hot, by removing their shirts and going bare bodied. By doing this, they expose themselves to more mosquito bites. On help-seeking behavior by the participants when sick, table 3 shows that more persons 150 (52.6%) indicated going to medical laboratory occasionally for the diagnosis of their ill health. 42 (14.7%) respondents indicated not going to 41 laboratory. Oral interviews on some of the participant that indicated not going to laboratories revealed that this trend is either due to poverty, uncertainty of test, time consuming process and poor knowledge on the right steps to take when sick. Table 4 shows the preventive measures against mosquito bites by the participants. A greater number of the respondents indicated use of fan (21.70%) and environmental sanitation (16.70%). The use of insecticide treated bed nets (ITNs) was indicated by the least group o f the respondents 5(1.7%). The reason for this low patronage was not be unconnected with the level of awareness, availability and affordability(Macintyre et al.,2002; Olayemiet al., 2003). Also 5(1.7%) of the respondents indicated that they did nothing as regards prevention of malaria infection. This agrees with the findings of Onwujekwuet al (2005) and Zambia RBM report (2001).Onwujekwuet al (2005) after investigating on the use of ITNs in Achi, Oji river L.G.A, Enugu State, reported of a very low use of the net by the people, while Zambia RBM report (2001), stated that poor families live in dwellings that offer little protection against mosquitoes and are less able to pay either for effective malaria treatment or for transportation to a health facility. On methods of malaria treatment by the participants, table 5 revealed that a greater number (27.6%) of respondents buy anti-malarial drugs from chemist without prescription by a physician, 34 (12.0%) of the respondents consult the physician by attending hospitals, while 60 (21.1%) of the respondents depend solely a traditional medicine from traditional healers. These findings agree with the observations of Ezedinachiet al (1997) and Foster (1995). Ezedinachiet al (1997) working on perception of malaria infection by people in two rural communities (Awi and IkotEdemOdo) in Cross River state of Nigeria, reported that traditional medicines were initial treatment responses, while formal health sector was consulted only if home initiated measures failed. Foster (1995) 42 investigating on rural malaria in Gambia, reported that a large proportion of malaria patients receive some form of treatment in the home or community without ever making contact with the formal health services. Of special interest is the revelation that few individuals (4.2%) indicated doing nothing at all to manage malaria. Whether they do not suffer the effects of the disease was not investigated. But it has been proved that some inherited disorders of haemoglobin such as sickle cell confer a reasonable degree of resistance against malaria to certain groups of individuals and that those individuals who are heterozygous for haemoglobin (AS) suffer malaria less frequently and less severely than normal individuals (Olumeseet al, 1997). An attempt was made to relate the management practices of the people with educational qualification possessed by individuals. It was interesting to observe the highest percentage of those who attend hospitals to treat malaria were those processing secondary (26.5%) and tertiary education (38.2%). On the other hand, the highest percentage of those who buy anti-malarial drugs over the counter and those who use traditional medicine possessed informal and primary education. Again, the highest percentages of those who make use of traditional medicine were those possessing informal (57.1%) and primary education (21.7%). It was also observes that the lowest percentage of those who attend hospitals, possessed informal education (14.7%) (Table 6). From the foregoing, it is possible to conclude that the higher the education level of an individual, the better the malariamanagement practices. The level of education an individual possess directly improves the awareness of the individual to disease conditions.In this era of roll back malaria, this particular observation has emphasized the need for the people to be more aware of the disease through functional education and public enlightenment program.Health education therefore becomes very important to teach people the simple relationship between mosquito bites and malaria, the need 43 to keep their environment clean and tidy, destroying anything that may become a conducive breeding site for malaria vectors and the current trends in the effective management of malarias. For a holistic control of malaria, therefore, everybody, that is, individuals, private agencies and government should be involved. CHAPTER 6 SUMMARY AND RECOMMENDATION 6.1 Summary This research set out to determine the prevalence of malaria infection among members of Ndiegoro community, Aba South L.G.A., AbiaState, attending hospital and to ascertain their management practices. The prevalence of malaria parasites was done using both thick and thin blood film microscopy on those attending local hospitals. On the knowledge of management practices of the people, qualitative data was elicited using structured questionnaire. It was also part of the objectives of the research to determine association (if any) of the level of education and method of treatment of malaria adopted. Similarly, age-specific and sex-specific prevalence were observed. The overall prevalence of malaria was found to be 51%. All the cases of malaria diagnosed were infection of Plasmodium falciparum. It was found that the preventive measures against mosquitoes bites adopted by the 44 community includes: door and windows screen, untreated bed nets, ITNs, mosquitoes repellent, environmental sanitation, using fan, insecticide spray, and chemo-prophylaxis. Also the malaria treatment methods by the people of the community include: the use of traditional medicine, the buying of anti-malarial from chemist without prescription of a physician, and attendance of local hospitals. Some people combine various management practices out of ignorance and poverty. Based on these findings and observations, the following recommendations are for the study community and for the general purpose of making significant difference in the bid to contain malaria in endemic areas. 6.2 Recommendation for Ndiegoro Community 1. A good percentage of the people buy anti-malarial drugs from shops to treat malaria. The World Health Organization (1999) in one if its studies observed that the management of malaria in the homes could be markedly improved by the use of genuine blister package doses of anti-malarial. The same study revealed that prepackaging of antimalarial ensures compliance with full course of treatment and drug management resulting in reduced cost of treating malaria and reduced waiting times in the drug dispensaries. Unfortunately fake drugs in shops markets are limiting this important malaria control practice. It therefore becomes necessary that government at all levels, market unions and pharmaceutical societies should work closely and win the war against fake drugs for the special benefit of the health of the poor. 2. There is need to educate the community on the appropriate practices in the management of malaria. This is necessary since a good number of the people are either uneducated or poorly educated. Therefore, 45 adequate sensitization through electronic media, seminars and workshops in advocated. 6.3 Recommendation for reducing malaria burden For effective control of malaria including prevention and treatment, the government at all levels should muster the political will to implement the existing tools towards the eradication of malaria. To achieve this, the following recommendations are made: 1. Related studies to this research work should be sponsored within regions with special interest in rural communities. These will reveal the patterns of the diseases within an area and enable use of the most effective control measure, thus improving the health of the people. 2. It has become increasingly appreciated that multi-sectorial approach to disease control is a better approach than monosectorial. It therefore becomes imperative that various sectors of the government should be adequately educated to understand the roles each can play in disease control. For instance. Ministries of Agricultures, Natural Resources, Housing and Environment and others should be made to closely interact with the ministry of Health so as to appreciate the impact their involvement can make in health of the people. Such roles by other ministry should center on poverty eradication. 3. Since ignorance is still an obstacle against the control of malaria, it is important to recommend that adequate airtime is given to malaria control programs in the electronic media, so that people especially the rural dwellers will know how to prevent and manage the disease. 46 4. Since malaria is endemic in Nigeria and constitutes a greater percentage of outpatients consultation in hospitals, it is recommended that free medical services should be provided for malaria patients. This will definitely make the difference in reducing the burden of the disease among the citizenry. 5. Since Plasmodium falciparum was the only Plasmodium parasite found prevanlent in the study, it is safe to recommend the use of easy-to-perform rapid immunodiagnostic techniques in the diagnosis of malaria. The usage is recommended because results obtained from such diagnosis could be quite reliable in the treatment of the infection. 6. In this era of “Roll Back Malaria” whose approach appears to be mainly preventive, using insecticide treated bed nets (ITNs) to limit both the mosquito population and man-mosquito contact, it is necessary it emphasize the need to make chemoprophylaxis and chemotherapy as important as the use of ITNs. This has become imperative since the long-term effects of the mosquito vector and man is yet to be fully known. It is therefore recommended that equal emphasis be paid to both the development and use of ITNs and anti-malarial drugs. In conclusion, an integrated approach which combines attacking both the parasite and vectors of the diseases with proper environmental management as well as positioning man to his roles in diseases control will definitely lead to a reduced burden of malaria and increase economic development of the people. 47 REFERENCES Adams, A.R.D. and Macgraith, B.G. (1985).Clinical Tropical Disease.8th Edition Adams, A.R.D. and Mcgraith, B.G. (1985).Prentice Hall International Inc. Afolabi, B.M., Sodeinde, O. and Audu, R.U. (1997): Malaria in Early Infancy on the Atlantic Cost of Lagos, Nigeria.The Nigerian Journal Medical Research 1(2): 32-36. Aribodor, D.N., Njoku, O.O., Eneanya, C.I., and Onyali, I.O. (2004). Studies on The Prevalence of Malaria and Management Practices of the Azia community in Ihiala L.G.A., Anambra State, South-East Nigeria.The Nigerian Journal of Parasitology24:33-38. Asianya, V.N., Agomo, P.U., Mafe, C.A., Akindele, A.G., Agomo, S.K., Aina, C.O., Okoh, H.I., and Omuruyi, S.O. (1999).Evaluation of a new chromatographic test for rapid diagnosis of Plasmodiumfalciparum malaria in Nigeria.The Nigerian Journal of Parasitology 20:19-26. 48 Asindi, A.A., Ekanem, E.E., Ibia, E.O. (1993). Upsurge of Malaria Related Convulsion in a Paediatric Emergency Room in Nigeria: Consequences of Emergence of Chloroquine Resistant Plasmodium falciparum. Tropical medicine 45:100-113. Attapeu Provincial Health Service (2003). A Survey Report of Knowledge, Attitude And Practice (KAP) On Malaria. The ACT- Malaria Information Resources I.D : BIB-1112 Awash, T. (2000).Homing in the Heart of RBM Movement. WHO/RBM News 1:2 Ayalde, J. and Oliver, M. (1990).Malaria. V.B.C. Tropical Disease Paper No.1. page 15-19, TainaLitwak Anne Masters Design, Inc. Chukwuocha, U.M., Ukaga, C.N., Dozie, I.N.S., Nwoke, B.E.B and Breiger, W.R., Nwankwo E.; Ezike, V.I, Sexton, D.; Breman, J.G; Pakes, K.; Ekanem, O.J. and Robinson, T.(1997). Social And Behavioural Baseline for Guiding Implementation of an Efficient Trial of Insecticide-Treated Bed nets for Malaria Control in Nsukka, Nigeria. Int’l Quarterly Journal of Community Hlth. Ed. 16:47-61 Cheesbroughu, M.(1998). District Lab. Practice in Tropical countries. Part 1. Cambridge Univ. Press U.K 239-258 49 Clark, I.A. (1987). Cell-mediated Immunity in Protection and Pathology of Malaria.Parasitol.Today 3: 300-305 Cooker, HAB, Chukwuani CM, Ifudu HD, Aina BA (2001). The Malaria Scourge.Concept in Disease Management.Nigeria J.Pharm. 32:19-48 Dick, B. (1985).The Impact of Refugees on the Health Status and Health Services of Host Communities. Comparing Bad with Worse? Disaster 9:256-269 Eneanya, C.I.(1998).Seasonal Variation in Malaria Episodes Among Residents in a semi-urban community in southeast Nigeria.The Nigeria Journal of Parasitology.19:39-43. Erhum, W.O. and Osagie, A. (2004). Management of Malaria by Medicine Retailers in a Nigeria Urban Community.Journal of Health & Population in Developing Countries (ISSN 1095-8940). Federal ministry of Health (2001). National Strategic Plan for Roll Back Malaria.Federal Ministry of Health, Abuja, Nigeria; 2001. Fleck, S.L. and Moddy, A.H. (1988).Examination of blood for malaria and other parasites. In Hematology (Williams NWJ, Bentler E, Erster AJ and Lichtman MA, eds) 4thed, McGraw-Hill, New York PP 17031706. Foster, S. (1995). Treatment of malaria outside the formal health services 50 J. Trop. Med. Hyg. 98:29-344. Garnham, P.C.C. (1971). Progress in Parasitology,TheAtlones Press, London. Gilles, H.M. (1999). Protozoal Diseases. Book aid International. Oxford Univ. Press.Inc. New York. Ike, I.M. (2000) Malaria Incidence in Abakaliki and Effects on SocioEconomic Development of the Area.The Nigerian Journal Parasitology Book of Abstracts.2000:12. Ike, I.M. and Udem, F.O. (2008) Prevalence of Malaria Infection in Patients Studied at Mater Misericordia Hospital, Afikpo, Ebonyi State Book of Abstracts, Parasitology and Public Health Soc. Of Nigeria.P43 Iweze, E.A. (1987). The Patent medicine store: Hospital for the Urban Poor.In the Urban Poor in Nigeria. Edited by MakinwaOzo, A.O., Ibadan, Nigeria: Evans Bro. Ltd; 1987: 317-32. Jaine, A. and Michael, O. (1990) Malaria, VBC Tropical Disease Paper. No. 1.page 3. Kombila, M. (1994). Focus on Recent Discoveries Concerning the Epidemiology of Malaria in the Africa Region.Malaria and Infections diseases in Africa.1:10-13. Kondrachine, A.V., and Trigg, P.T. (1997). The Current Global Malaria 51 Situation 11-12 in I.W. Sherman (ed.). Malaria: Parasite Biology, Pathogenesis and Protection. ASM Press. Washington D.C., U.S.A Krogstad, D.J. (1996). Malaria is An Emerging Disease. Epidemiological Review.18:77-89. Kwiatkowski, D. (1995). Malaria Toxins and the Regulation of Parasite Density.Parasitol. Today 11:206-212. Legesse Y, Tegegn A., Balachew T. Tushune K. (2007). Knowledge, Attitude and Practices (KAP) about malaria and its preventive measures among households in urban areas of Assosa zone, Western Ethiopia. Ethiopian Journal of Health Development 2007: 21(2) 157165. Levy, M.D., Bushkila, Gladman, D.D. (1991). Pregnancy outcome following first trimester exposure chloroquine. AmericanJournal of Perinatology. 8:174-178 Lindsay, S.W., Birley, M.H. (1996). Climatic change and Malaria Transmission.AnnalsTropicalMedicineParasitology 90:573-88. Malakooti, M., Biomndo, K., Shanks, D.C., (1998). Re-emergence of Epidemic Malaria in the Highlands of Western Kenya.Emerging Infections Disease. 4:671-6 Matur, B.M., Azare B.A. and Ugbong, I. (2001). The Prevalence of 52 Asymptomatic Malaria ParasitaemiaAmongst Undergraduate Students of University of Abuja. The Nigeria Journal of Parasitology.22:4452. Mbanugo, J.I, and Ejims D.O. (2000).Plasmodium Infections in Children Aged 0-5 years in Awka Metropolis, Anambra State. Nigeria. TheNigeriaJournal Parasitology 21:55-59. Miller, L.H., Good, M.F. &Kaslow, D.C. (1997). The Need for Assays Predictive of Protection in Development of Malaria Blood Stage Vaccines.Parasitol.Today 13: 46-47. Miller, L.H., Mason, S.J., Clyde, D.F., and McGinniss, M.H. (1976). Resistance Factor to Plasmodiumvivax:duffy genotype Fyfy. New England Journal of Medicine,295:302-304. National Centers for Zoonotic, Vectors-borne, and Enteric Diseases. National Institute of Health (NIH, 2001) NIH News Release-Malaria Research and Training Benefits Global Community. Nebe,O.J., Adeoye, G.O., Agomo, P.U. and Mosanya, M.E. (2002). Malaria in Coastal Area of Lagos State, Nigeria: A Survey of Perception and Practice Among the Mothers /Caregivers of Children under five years old. Nigeria Journal of Parasitology.23:69-80. Obi, C. (1997): Coping with treatment failure in malaria. Proceeding of the 53 Update Symposium on Rational use of Anti-Malaria Drugs. Organized by May and Baker Nigeria Plc. Lagos Airport Hotel, Ikeja. 7-8. Obiukwu, M.O. and Okwuonu, E.S. (2008) Prevalence of Malaria and its Effects on Socio-Economic Development as well as Management Practices Adopted in Abba, Njikoka L.G.A. Anambra State, Nigeria.Book of Abstract of Parasitology and Pub.Health Soc.Of Nigeria.P.63. Olarenwaju, W.I., Johnson, W.B.R. (2001): Chloroquine – Resistant Plasmodium falciparum malaria in Ilorin, Nigeria: Prevalence and Risk factors for Treatment Failure. Afr. J. Med. Sci. 2001;30:165-169. Olumese, P.E., Adeyemo, A.A., Ademowo, O.G., Gbadegesin, R.A., Sodiende, O. and Waler, O.(1997). The Clinical Manifestations of Cerebral Malaria among Nigerian Children with Sickle Cell Trait. Molecular Medicine: 3:581-92. Onwujekwe, O.E., Uzochukwu, B.S.C., NkoliEzuma, and Shu, E. (2005). Increasing Coverage of Insecticide-treated nets in Rural Nigeria: Implications of Consumer Knowledge, Preferences and Expenditures for Malaria Prevention. 54 Onwuliri, C.O.E. (2008). Malaria in the middle Imo River Basin of Nigeria: Distribution of Plasmodia Species and Common Clinical Symptoms in Rural Community of Imo State.Book of Abstracts of Parasitology and Public Health Society of Nigeria.P.61. Oyewole, I.O. and Ibidapo, A.C.(2007). Attitude to Malaria, Prevention, Treatment and Management Strategies Associated with the Prevalence of Malaria in a Nigerian Urban Center. African Journal of Biotechnology vol. 6(21), PP. 2424-2427, 5 Nov., 2007. ISSN 16845317 (c) 2007 academic journals Payne, D. (1993). A lens too far.Transaction Royal Society Tropical Medicine and Hygiene. 87:496 Pelletier, D.L., Frongillo, E.A., Jnr., Schroeder, D.G., Habicht, J.P. (1995); The Effects of Malnutrition on Child Mortality in Developing Countries. Bulletin of the World health Organization. 73(4) 443-448. Quintana, M. et. al. (1998). Malaria Diagnosis by Dipstick Assay in Honduran population with Co-endemic Plasmodium falciparum and P. vivax.American Journal of Tropical medicine and Hygiene.59:868871. Salako, S.A. (1986). Malaria in Nigeria: Proceedings of the conference on 55 Malaria in Africa. American Institute of Biological Sciences, Eleventh Street, N.W. Washington, D.C. 131-141. Salako, L.A. (1997). Malaria the Unending Saga. Proceedings of the update Symposium on Rational use of Anti-Malaria Drugs, Organized by May and Baker Nigeria PlcIkeja, Nigeria 13-20 Salwa, M.E. Abd/El-Gayoum, El-Amin.El-Rayah, Hayder. A. Giha, and Abd El-Karim, A. El-Feki, Malaria in Man-Made Malarious Area in Khartoum State, Sudan http://www.Sjph.net.sd/files/vol4: il/p199209.pdf Schmatz, B. and Schaffer, J. (1991).Anti-parasite agents. Annual Report and Chemistry: 26:161-70. Sherman, I.W. (1998). A Brief history of Malaria and Discovery of the Parasite’s Life Cycle. In Malaria: Parasite Biology; Pathogenesis and Protection. I.W. Sherman (ed) ASM Press. Washington. D.C. Snow, R.W., Guerra, C.A., Noor, A.M., Myint HY, Hay SI (2005). The Global Distribution of Clinical Episode of Plasmodium falciparum Malaria. Nature 434:214-217. Steketee, R.W. (2001). The Burden of Malaria in Pregnancy in Malaria- 56 Endemic Areas.American Journal of Trop. Medicine and Hygiene (1,25): 28-35. 2001. 64 Sinnis, O., and Sim, B.K.Z., (1997). Cell Invasion by the Vertebrate Stages of Plasmodium.Trend microbiology.5:52-58. Taylor, P., Mutambu, S.L., (1986). A Review of the Malaria Situation in Zimbabwe with Special References to the Period 1972- 1981.Transaction Royal Society Tropical Medicine Hygiene. 80:12-19 Tracy, J., and Webster, L. (1996) Drugs used in the Chemotherapy of protozoal infections. In J. Hardman, P. Molinoff, R. Ruddon and A. Gilman (ed), Goodman and Gilman’s. The Pharmacological Basis of Therapeutics.Peramon Press. N.Y., U.S.A. Ukoli, F.M.A. (1990): Introduction to Parasitology. University of Ibadan, Press. Ukpai, O.M. and Ajoku, E.I. (2001). The Prevalence of Malaria in Okigwe andOwerri Areas of Imo State. The Nigeria Journal Parasitology.22:43-48. Ukpai, O.M. and Amaechi, C.E. (2008). Knowledge, Attitude and of 57 Practices Amongst Mothers And Care-givers in Aba South L.G.A, Abia State, Nigeria. Book of Abstract, Parasitology and Public Health Society of Nigeria. Sept 2008. P 66. UNICEF (2000) Roll Back Malaria. A Global Partnership Rolling Back Malaria. United Nations International Children’s Fund (UNICEF). Usip, C.P.E. and Opara, K.N. (2008). Longitudinal Study of Malaria Infection in Uyo Urban, Nigeria.Book of Abstract, Parasitology and Public Hith Soc.Of Nigeria.P 25. Uzochukwu, B.S.C, Onwujekwe, E.O., Onoka, C.A. and Ughasoro M.D. (2008). Rural-Urban Differences in Maternal Responses to Childhood fever in South-East Nigeria. PLOS ONE 3(3): el788. doi:10.1317/journal-pone.000178. Warrell, D.A. (1987).Pathophysiology of Severe Falciparun Malaria inMan Symposia of the British Society of Parasitology (vol 24) Parasitology 74: 553-576. Warell, D.A., Looareesuwan, S., Philips, R.E., White, N.J., Warrell, M.J., 58 Chapel, H.N., Areekul, S., and Tharavanji, S. (1986). Functions of the Blood Cerebrospinal Fluid Barrier in Human Cerebral Malaria. Rejection of The Permeability Hypothesis. Am. J. Trop. Med. Hyg.35:882-89. Wilcox, M., Bjorkman, A., Brohult, J., Pehrson, P.O., Rombo, L. and Bentsson, E. (1983). A case control study in Northern Liberia of Plasmodium falciparum malaria inhaemoglobin S. and betathalassaemia traits, Annals Tropical Medicine and pathology 77:239246. Wolfe, M.S., and Corders, J.F., (1985). Safety of Chloroquine in Chemosuppression of Malaria During Pregnancy. British Medical Journal. Clinical Research 290: 1466-67. World Health Organization (1991a).Basic Malaria Microscopy.Part 1. Learner’s Guide, WHO, Geneva. World Health Organization (2001).Specification for Netting Materials. Report of an Informal Consultation .WHO, Geneva. 8-9 June, 2000. WHO/CDS/RBM/2001:28:1-8. WHO (2001).The Use of Antimalarial Drugs. Report of WHO Informal Consultation. Geneva. WHO, 2001, (WHO/CDS/RBM/2001.33) 59 WHO (1993). Tropical Disease Research: Progress 1991-1992. 11th Program report of the UNDP/WORLD BANK/WHO Special Program for Research and Training in Tropical Disease.WHO Bull.15-29. WHO (1996a).Tropical Disease Control.Malaria in the World,1996. World Health Organization Unpublished document (CTD/AT/196.12. World Health Organization (Action Program (1986a) Severe and Complicated Malaria. (Trans. R. Soc. Trop. Med. Hyg. 80(suppl). 1-50 World Health Report (2002): Reducing Risks, Promoting Healthy Life. Geneva. World Health Organization, 2002. World health Organization (1986b). The Development of Artemisinin and its Derivatives. WHO/TDR/CHEMAL/ART 86:1-30. World Health Organization (1997b). Management of uncomplicated malaria and the use of Antimalaria Drug for the protection of travelers. World Health Organization unpublished document WHO/MAL/96 World Health Organization (1997c) ArtesunateRecolaps a Life Saving Intervention.WHO/TDR News 53:2-3. World Health Organization (2001).Specifications for Netting Material. 60 Report of an Informal Consultation WHO, Geneva 8-9 June 2000. WHO/CDS/RBM/2001:281-8 WHO (1998). Malaria: Know the facts, World Health Organization News Letter 13 (1): 6-7. WHO (1998).Examining blood for malaria parasites. Bench Aids for the Diagnosis of Malaria, 1-8 plates.World Health Organization. WHO (2000). Roll Back Malaria. Promise for Progress. Roll Back Malaria Cabinet Project, WHO Geneva, 4-6. WHO (2003a). The African Malaria Report 2003, Geneva, World Health Organization/United Nations Children’s Fund 25th April 2003 (WHO/CDS/MAL/2003.1093). WHO (2005). World Malaria report 2005, Geneva World Health Organization WHO/HTM/MAL/2005.1102. QUESTIONNAIRE NNAMDIAZIKIWEUNIVERSITY AWKA DEPARTMENT OF PARASITOLOGY AND ENTOMOLOGY TOPIC: The prevalence of malaria infection among members of Ndiegoro community, Aba South L.G.A., AbiaState, attending hospital and to ascertain their management practices. 61 Dear Respondents, The above study is being carried out by me, of the above department, NnamdiAzikiweUniversity, Awka. Kindly assist me by ticking (√) appropriately in the columns provided. Please be very honest in your response to each item. Your co-operation is highly appreciated. All information will be treated in confidence. SECTION A: PERSONAL DATA 1. Sex: Male [ ] 2. Age (Please specify) [ ] 3. Educational Qualification: 4. Female [ ] a. Informal [ ] b. Primary [ ] c. Secondary [ ] d. Tertiary [ ] Marital Status: Single [ ] Married [ ] SECTION B. 5. How often is malaria diagnosed? a. very often [ ] b. occasionally [ ] c. none at all [ ] SECTION C 6. How do you mosquitoes bite prevent? a. door and window screen [ ] b. untreated bed nets c. Insecticide treated nets(ITNs) [ ] [ ] 62 f. d. cover cloth [ ] e. mosquito repellant [ ] environmentalsanitation [ ] g. using fan [ ] h. insecticide spray [ ] i. chemoprophylaxis [ ] j. none [ ] SECTION D 7. How do treat malaria you? a. buy antimalarial drugs from chemists [ ] b. attend hospitals [ ] c. use traditional medicine(herbs) [ ] d. trad.med and attend hospital [ ] e. antimalarial drugs and trad. Med [ ] f. antimalarial drugs and attend hospital [ ] g. antimalarial drugs &trad. Med.& attend hospital [ ] h. none [ ] APPENDIX MINITAB OUTPUT Chi-Square Test (Dependence of prevalence on age) Expected counts are printed below observed counts 0-5 No infec No uninf Total 26 9 35 17.73 17.27 6-10 25 20.27 15 19.73 40 11-15 17 16.21 15 15.79 32 16-20 14 24 38 63 19.25 18.75 21-25 16 17.23 18 16.77 34 26-30 10 11.65 13 11.35 23 31-35 18 16.72 15 16.28 33 36-40 11 14.19 17 13.81 28 41> 15 18.75 22 18.25 37 Total 152 148 300 Chi-Sq= 3.854 + 3.958 + 1.105 + 1.135 + 0.038 + 0.039 + 1.433 + 1.472 + 0.087 + 0.090 + 0.235 + 0.241 + 0.098 + 0.101 + 0.716 + 0.735 + 0.749 + 0.769 = DF = 8, P-Value = 0.032 16.855 Interpretation: Since the p-value(0.032) is less than 0.05(level of significance) we reject the hypothesis that prevalence of..... does not depend on age. APPENDIX 2 Chi-Square Test(Dependence of prevalence on sex) Expected counts are printed below observed counts No_infecNo_uninf Total Male 88 62 76.50 73.50 Female Total 150 65 76.50 85 73.50 150 153 147 300 Chi-Sq= 1.729 + 1.799 + 1.729 + 1.799 = DF = 1, P-Value = 0.008 7.056 Interpretation: since the p-value is less than 0.05, we reject Ho and conclude that prevalence 64 depends on sex.
© Copyright 2025