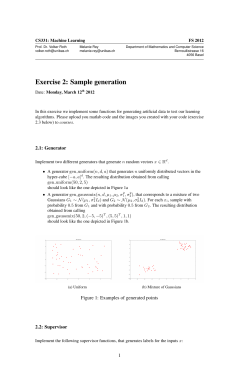
ORGANIC CHEMISTRY RESEARCH GENERAL SAFETY MANUAL
Organic Safety Manual 12th Edition, created 22.09.2014 by Simon Woodward ORGANIC CHEMISTRY RESEARCH GENERAL SAFETY MANUAL No one may work in these laboratories unless they have read and understood these notes, and agree to abide by the general recommendations, protocols and prohibitions contained therein. The Safety Form at the end of this manual must be completed and countersigned by the research supervisor, and given to the Section Safety Officer (Prof. Simon Woodward) before starting work. 2 CONTENTS Page 1 A. SUMMARY OF SAFETY RULES B. SAFETY LEGISLATION 1. The Health and Safety at Work Act 2. Control of Substances Hazardous to Health 2 2 2 C. RISK ASSESSMENTS 1. 1st–3rd Year Undergraduate Laboratory Work 2. 4th Year Undergraduate Laboratory Work (Projects) 3. Postgraduate Research Assistants 4. Members of Staff 5. Making Risk Assessments 6. The COSHH Database 3 3 3 3 5 5 9 D. EMERGENCY PROCEDURES 1. The Emergency Alarm 2. Types of Fire and Fire-fighting 3. Safety Stations 4. Breathing Apparatus 5. Basic First Aid 6. Accident Reporting 10 10 11 12 12 13 16 E. RULES GOVERNING USE OF THE CHEMISTRY BUILDING 1. Normal Working Hours 2. Outside of Normal Working Hours 3. Building Services 4. Overnight or Unattended Experiments 16 16 17 17 18 F. SAFETY IN THE LABORATORIES 1. Good Chemical Laboratory Practice 2. Additional Safety Measures 3. Preventing Accidents 4. Common Causes of Personal Injury 5. Tidyness 6. Communal Facilities 18 18 19 20 23 25 26 G. CHEMICALS AND SAFETY 1. General Considerations 2. Chemical Toxicity 3. Laboratory Hazards Boards 4. Gas Cylinders 5. Liquid Nitrogen 6. Detoxification and Disposal 28 28 28 32 32 33 34 H. BIBLIOGRAPHY 39 I. EMERGENCY TELEPHONE NUMBERS inside back cover 1 A. SUMMARY OF SAFETY RULES 1. Safety glasses must be worn at all times in the laboratories. Laboratory coats are essential wear for Organic experimental work. Bare arms and bare legs are not allowed for experimental work. 2. In laboratories , note the position of fire extinguishers, fire blankets, etc. 3. No one may carry out experimental work whilst alone in the laboratories. 4. COSHH assessments must be completed before preparative experiments are attempted. 5. Put compound hazards on whiteboards outside labs on a daily basis. Use red pen for stench chemicals 6. Eating, drinking, smoking, or the use of radios, cassette players etc. are forbidden. 7. Overnight or unattended experiments must carry a completed overnight card. Risk experiments must have been independently checked, and the card countersigned by the research supervisor or his/her nominee. 8. All unauthorized experiments are expressly forbidden. 9. All persons present in the Department out of hours (08.30–18.00, Monday–Friday) must sign the 'out-of-hours' register. 10. The fire alarm is tested on Monday mornings at about 8.55 am. Unless the alarm is not switched off after about 30 seconds, you need not do anything. At all other times if the alarm goes off, evacuate the building in an orderly fashion immediately. These rules are in place for your own protection. Contravention of any rule is regarded as a serious offence, and disciplinary proceedings will follow. 2 B. SAFETY LEGISLATION All work in the Chemistry Department is subject to two Acts of Parliament. Compliance with this legislation is monitored by the Health and Safety Executive (the HSE) who have a large number of inspectors spread around the various regions of the country. An HSE Inspector has wide-ranging powers; these include the ability to shut-down a place of work or to bring a criminal prosecution for infringements of the Acts. We are all therefore obliged to obey both the terms and spirit of each Act. 1. The Health and Safety at Work Act (1974) This Act places duties on all employed persons to take reasonable care of their own health and safety and the health and safety of others who may be affected by what they do, or omit to do. In addition every employee has a duty to co-operate with their employer and must not intentionally or recklessly interfere with or misuse anything provided in the interest of health, safety, or welfare. Thus, everyone bears a statutory responsibility for taking due care in the laboratory, for observing appropriate precautions, and taking necessary preventative measures when carrying out operations. Remember that there is always an element of danger present in a laboratory. You will be in frequent contact with potentially dangerous materials or equipment, careless or improper use of which can result in a serious accident, even physical injury. In research work it is particularly important to try and anticipate problems; make sure that you are always properly briefed. Do your homework! If in doubt – ask! 2. Control of Substances Hazardous to Health Regulations (COSHH, 1990) Basically, these Regulations redefine the way in which toxic materials can be handled at work, and it is a criminal offence for any work using such materials to proceed without a proper assessment first having been made. The object of an assessment is the elimination or adequate control of risks to health. Risk is a function of both hazard (potential of a substance to impair health) and exposure potential (extent to which a substance may enter, or come into contact with, the body). Broadly speaking: RISK = HAZARD x EXPOSURE POTENTIAL 3 C. RISK ASSESSMENTS 1. 1st-3rd Year Undergraduate Laboratory Work (Set Experiments) These students obtain all of their essential information from the laboratory manuals with which they are supplied. The experiments have been assessed by the member of staff in charge of that laboratory, and notable risks and the precautions to be taken are recorded in the individual experiments in the student's manual. No separate assessment is necessary since the assessment is implicit in the experimental detail given to the student in the manual. ● However, Demonstrators must save undergraduates from the foolishness of their own actions by constant, careful surveillance of the teaching laboratory and by rendering practical help and assistance whenever necessary. 2. 4th Year Undergraduate Laboratory Work (Projects) Each of these experiments must be separately assessed using the standard COSHH assessment grid. Project students should be treated as in 3(a) - i.e. as inexperienced research assistants, and the project supervisor (or nominee) must check and sign the assessment before the student attempts the experiment. High risk experiments [i.e. Category A, see 3(a) below] must be directly supervised. Postgraduates should keep project students under constant and careful surveillance and give help when necessary; the project supervisor (or deputy) will be present in the laboratory only for limited periods of time. Undergraduates are not allowed to work when the laboratory is not manned by postgraduates. 3. Postgraduate Research Assistants ● Failure to carry out COSHH assessments will be treated as a serious offence, and will lead to immediate suspension from laboratory work. This applies to all personnel, whatever their status. (a) First Year Postgraduates Although the B.Sc./M.Sci. degrees (or equivalent) indicate a recognized qualification in chemistry, new postgraduates are nevertheless relatively inexperienced in experimental work. They are expected to do their own assessments which the supervisor (or nominee) should check and sign 4 before the experiments are started. This procedure should be followed normally for the first 3 months (e.g. conveniently from 1st Oct to 31st Dec) or until such time as the supervisor decides is necessary. Procedure 3(b) is then followed. However, assessments also place experiments into one of four categories A-D, depending on the risk; these categories have the following meanings: A B C This activity must be directly supervised. The supervisor's advice and approval must be sought before the task is started. The work involves risks requiring careful attention to the safety related aspects of it. The worker has been trained in the task and has demonstrated competence. D Tasks in this category carry no undue risks. (In preparative work, very few reactions fall into this category). Category A is for experimental work that potentially has a high risk because one (or more) of the reactants is especially hazardous, or the procedure itself may be hazardous. This includes: ● ● All work listed under the Standard Protocols section of the COSHH grid (e.g. Pyrophorics, Hydrogenation, Peroxides, Ozone, etc.) Any work involving the use of carcinogenic materials (e.g. alkylating agents, benzene, etc.). ● ● ● Any work involving the use of inorganic cyanides or other highly toxic substances. Materials listed as explosives as well as some powerful oxidising substances. Reactions run in sealed or pressurized systems. If Category A is pertinent, then your supervisor must arrange for you to be trained in the procedure under direct supervision. Information can be found on the departmental website and you must complete your Record of Safety Competence and have it signed before you start the experiment. Once you have been trained in the appropriate method to your supervisor's satisfaction, then for that type of work in future the Risk Category may be downgraded to Category B. Separate training is necessary for each type of circumstance in Category A before it may be downgraded to Category B for future work (i.e. training in handling pyrophorics does not equip you to handle liquid ammonia safely!). However, once the research project is well underway, most of your work will be in Risk Categories B or C. ● Other, more experienced personnel in the laboratory should take it upon themselves to keep an eye on new postgraduate students and give help and advice when necessary. It is recognized that the "supervision" by the research advisor can never be constant. 5 The research supervisor is expected to check research coworkers COSHH assessments periodically and sign the laboratory notebook to indicate that the supervisor is aware these experiments are being conducted. (b) Other Postgraduates and Post-doctoral Assistants Each experiment must be properly assessed and the assessment recorded before the experiment is attempted. The research supervisor is expected to check these assessments periodically and sign the laboratory notebook to indicate that such a check has been made. Clearly, if the supervisor feels that the assessment is deficient in any way he should discuss the matter with the person concerned and any problems should be rectified. Category A tasks [see Section 3(a)] may only be performed without direct supervision if you have already received training in that task and are deemed competent. ● Even postdoctoral assistants cannot be expected to know everything; do not be too proud to seek out expert help! 4. Members of Staff (a) Members of the academic or technical staff engaged on non-routine experimental work are also expected to carry out the full assessment procedure. (b) Members of the technical staff engaged on routine duties (i.e. nmr services, mass spectrometry and stores personnel) which involve the handling of chemicals should each have received a copy of an assessment of their duties. Separate assessments of their daily tasks are not necessary, but any change in their job routine, or any problems, should be reported to the Safety Officer so that the appropriate modifications to the assessment can be made. 5. Making Risk Assessments ● All persons engaged in preparative organic chemical work must use the laboratory notebooks supplied by the Main Stores. These notebooks are printed on every left-hand page with a blank assessment grid (see Examples 1 and 2, pp. 7–8). Fill in the upper part of the grid with the names, quantities, and hazards of all of the chemicals to be employed in that experiment. The hazards information is most conveniently obtained from the COSHH computer database (http://www.nottingham.ac.uk/chemistry_internal/safety/coshh/index.php). Or consult the Aldrich chemicals catalogue. For most chemicals you will find the EEC risk phrase, signified for example by R 36/37/38. These numbers can be interpreted by referring to the page ‘Hazardous Products’ near the front of the Aldrich catalogue. 6 ● The database also automatically selects the appropriate handling protocols (i.e. fumehood, rubber gloves, etc.) for each chemical. Hence, the protocol to be adopted for the experiment is the most stringent among all those listed for the chemicals in question. The working protocol is selected in the COSHH assessment grid by checking the appropriate boxes. ● If the hazards for one (or more) of the reagents is unknown (i.e. something new that you have just synthesised) then the minimum protocol is GCLP + fumehood + rubber gloves. (GCLP = good chemical laboratory practice – see p. 19). ● In the Working Practice part of the COSHH grid there are various other boxes that may need to be checked. For example, if one of the chemicals is butyllithium you must first have read the specific Safety Protocol on Pyrophorics (available on the departmental web page). Work with these is covered by a standard protocol, so you would need to check the “Inert Atmosphere” and “Pyrophorics” boxes. ● Next check the appropriate box for the Primary Containment of the reactants; if one of these descriptions does not fit your requirements, then give a brief description alongside one of the unattributed boxes, and check that box. Think – is the primary containment sufficient for the reaction in hand? ● The “Other Risks and Control Measures” and “Emergency Procedures” part of the grid are there to make you think about what other risks may arise from the experiment. Is the product or a by-product of the reaction hazardous? How can these risks be minimised? What should be done if the experiment goes wrong and the reaction mixture is released uncontrollably into the laboratory? You must plan ahead for such contingencies. To help you in making such decisions, two examples of COSHH Assessment grids are given on pages 7 and 8. ● The experiment must also be placed into one of four categories A–D, depending on the potential risk which dictates the level of supervision that is required (see p. 4). ● Repeated experiments do not require a full assessment (simply cross-reference to the previous assessment) provided that the previous assessment is within 5 pages in the same book and that no significant changes in reaction scale or method are being made. At the top corner of the right-hand page is a printed rectangular box for inserting your personal unique number for the experiment. This number should also be used for labelling spectra and other data generated by the experiment. 7 Example 1. Friedel-Crafts reaction of iso-butylbenzene (50 ml) with acetyl chloride (20 g) using aluminium trichloride (37 g), 0°C 20°C. Chemicals Quant. iso-Butyl benzene 50 ml Eye irritant, flammable Acetyl chloride 20 g Aluminium trichloride 37 g Working Practice (GCLP PLUS:–) Fume hood Rubber gloves PVC gloves Heavy gloves Screens Full face shield Dust mask Primary Containment: Inert atmosphere Spillage tray Standard Protocols: Pyrophorics Hydrogenation Peroxides Open Flask Flask + condenser Risk Category: Irritating to eyes, respiratory system and skin, highly flammable Irritating to eyes, respiratory system and skin, harmful, reacts violently with water UV light Diazomethane Liquid ammonia Ozone HF Multineck flask &c. Sealed flask / tube Scrubber for HCl evolved; final distillation at reduced pressure using an oil bath. Flush all spillages to drain with copious water. Other risks and Control Measures: Emergency Procedures: Hazards A B C D Signature: 8 Example 2. Methylation of methyl orsellinate (12.64 g) using methyl iodide (197 g), dry acetone (150 ml), and potassium carbonate (80 g); magnetic stirring and reflux for 12 h. Chemicals Quant. Hazards Iodomethane* Methyl orsellinate 197 g 12.64 g 150 ml 80 g Carcinogen, toxic, irritant Hazards not known Acetone Potassium carbonate Working Practice (GCLP PLUS:–) Fume hood Rubber gloves PVC gloves Heavy gloves Screens Full face shield Dust mask Primary Containment: Inert atmosphere Spillage tray Standard Protocols: Pyrophorics Hydrogenation Peroxides Open Flask Flask + condenser Highly flammable# Harmful by inhalation, skin contact or ingestion UV light Diazomethane Liquid ammonia Ozone HF Multineck flask &c. Sealed flask / tube None. All work-up procedures carried out in the fume hood; crude product carried through to the next stage. Evacuate laboratory; re-entry only by personnel wearing breathing apparatus and rubber gloves. Open lab. windows. Mop up spillage if possible and place swabs in a sealed jar – otherwise flush to drain with copious water. Other risks and Control Measures: Emergency Procedures: Risk Category: A B C D Signature: * Here it is assumed that the student is inexperienced in the use of alkylating agents (hence risk Category A). A person experienced in the use of alkylating agents would enter Category B. In this case, the experiment must only be conducted under direct supervision. # Flammability must be considered when deciding on apparatus design, method for heating (if applicable), and risks posed by nearby experiments. 9 6. The COSHH Database ● The COSHH Database can be accessed from the School of Chemistry’s website (http://www.nottingham.ac.uk/chemistry_internal/safety/coshh/index.php) ● The Database can be searched by chemical or owner to find out who has a stock of any of >12500 chemicals. For each chemical there is a complete hazard listing and details of good working practice and disposal procedures . Chemicals can be added by clicking on ‘Submit New Chemical’ and following the instructions. Please note that even if a chemical already exists on the database you must add your research group (by entering your supervisor’s name) as a new owner. ● It is vital that the Database be kept up-to-date and be free from error. Any discrepancies, errors or points of disagreement should be given in writing to Dr. Bertram (anna.bertram@nottingham.ac.uk). If you are in doubt, or have cause to suspect that the hazards entered for a given compound may be incorrect, then consult the Aldrich chemicals catalogue. For most chemicals you will find the EEC risk phrase, signified for example by R 36/37/38. These numbers can be interpreted by referring to the page ‘Hazardous Products’ near the front of the Aldrich catalogue. ● Keeping the data up-to-date is a simple routine. Proceed as follows:(a) If a chemical on your group's or laboratory's listing is used up but immediately re-ordered, then no action is necessary – unless the stock-holding has changed. # . (b) If a chemical is used up and not re-ordered, it should be deleted from your listing. The chemical name itself will not deleted from the database main file. (c) If a chemical not on your group/laboratory listing is ordered or taken from the stores,# then you must immediately add that chemical name to your listing. If you cannot find the chemical, first try the common synonyms – the database largely follows the naming protocols used in the Aldrich catalogue. (d) Your group or laboratory should up-date its chemical inventory regularly – say once every quarter. # Note: All commercial chemicals on receipt must be coded on the label (use heavy pencil in preference to a permanent felt tip marker) with your supervisor's initials, and the month and year of purchase (e.g. GP/11/08). This not only helps to establish ownership, but also gives an immediate indication of the age of any chemical. 10 D. EMERGENCY PROCEDURES 1. The Emergency Alarm (a) General ● The emergency alarm is a continuous-sounding siren. It is tested just before 09.00 every Monday. Alarm points are located generally near to a corner of the building, near the intersection of corridors and near the main staircase. The alarm can be activated by breaking the protective glass cover to the bell-push. If the fire alarm has to be sounded, the emergency call must be backed-up by a telephone message (Tel: 8888 - internal). ● All users of the building must familiarise themselves with the various exit routes from their working areas. All exit points are clearly marked, and must be kept clear of obstructions at all times. All fire doors must be kept closed and not wedged open. ● Be sure that you know the location of the nearest telephone, fire alarm, extinguishers, fire bucket, fire blanket and safety station for other equipment. (b) Emergencies and Evacuation If you discover an emergency situation, raise the alarm immediately. Do not try to contain the emergency situation unless it is within your competence to do so and you do not put your life at risk. Adopt a calm attitude and, unless you are a member of staff, evacuate the building by the nearest fire exit. ON HEARING THE FIRE BELL ● If practical, make all apparatus safe (e.g. turn off all heating sources). ● Close windows and doors on leaving, but leave the lights on. ● If you are the last person to leave a room or laboratory, alter the slide indicator on the door so that the GREEN sector is visible. (i.e. GREEN = room has been checked and completely evacuated).* Demonstrators must ensure that undergraduates in their class have left before they themselves evacuate the teaching laboratories. ● Evacuate the building. DO NOT USE THE LIFT. Assemble in the the paved area in front of the GG library (i.e. adjacent to Pope, GG library, and the lawn in front of Maths/Physics). Keep at least 20 metres away from the Chemistry building itself! ● Remain at the assembly point while routine checks are made;* do not re-enter the building unless told it is safe to do so by the Assembly Point Officer. The Assembly Point Officer 11 and the Safety Officers for the three sections of the Department will normally be wearing fluorescent yellow jackets for ease of identification. IF AN EVACUATION IS NECESSARY OUT OF NORMAL WORKING HOURS, A SENIOR PERSON PRESENT MUST TAKE THE RESPONSIBILITY OF CHECKING FOR ANY MISSING PERSONS. * Members of Staff have the task of checking that the evacuation has been completed. These procedures, which are based on a fire token system, are the subject of specific instructions and training. The door of every room in the Department is fitted with a slide indicator. Normally these show RED. After checking during an evacuation, the indicator is changed to GREEN to give an immediate simple indication that the room is empty of personnel. The indicators have no other purpose; do not fiddle with them and ensure that those for the rooms you use routinely show RED. On returning after an emergency evacuation, make sure that the indicator for the room you use is altered back to show the RED sector. 2. Types of fire and fire-fighting If in any doubt that the fire might get out of control, sound the fire alarm. Do not take personal risks. If practicable, tackle the fire with the fire fighting equipment available. Make use of the appropriate fire extinguisher (see below). Fires are classified according to British Standard EN2 (1992). The classification gives useful information to the emergency services. Electrical fires are not classified separately, but such equipment should first be disconnected from the mains: Class A Fires involving ordinary combustible materials such as wood, cloth, and paper are usually tackled with water or CO2 extinguishers. (Caution the force of a CO2 extinguisher fired into a confined space, such as a waste bin, may shower the firefighter with burning embers because of the back-draught! CO2 is often not effective for large fires because of reignition). Class B Fires involving flammable liquids such as petrol, paraffin, organic solvents, liquefiable solids, oils, greases and fats (CO2 may be effective for small fires – unless at a height, but re-ignition is a problem with larger fires. Foam and dry powder extinguishers efficiently extinguish large fires, but create a mess). Class C Fires involving gases – extinguish only after isolating the supply. Extinguishing before may cause an explosion. 12 Class D Fires involving burning metals and some of their compounds such as NaH and LiAlH4. (Special extinguishers are required with training for staff in handling combustible metals; do not use CO2 or water-based extinguishers). Fire extinguishers, fire fighting points, emergency stations and breathing apparatus sets will be clearly identified and must be kept clear of obstructions; these facilities must not be abused. Items of such equipment must not be removed (except for their intended use) without the express permission of an authorised member of staff. Inform Mr J G Gamble if extinguishers or breathing apparatus are used so that the supplies can be replenished. 3. Safety Stations The Safety Stations (one is located in C.32 and one in C.13) should contain the following items for emergency use: ● ● ● ● long and short handled brushes long handled squeegee mop and bucket sand bucket. ● ● ● ● hand shovel long handled tongs lidded metal bucket large polythene sheet (for flood protection) Items must be returned immediately after use. Report the absence of items to Mr. D. Toplis. 4. Breathing Apparatus Sets are available in the red-painted wooden cabinets located on the back stairs opposite lab. C.33. Instruction in the use of the breathing apparatus is given every October. If you miss this, or require a refresher, see Mr D. Toplis. Certain people should not use the breathing apparatus in an emergency (i.e. those with heart conditions, respiratory problems and those with beards or spectacles that prevent the face mask from seating properly). If there should arise an uncontrolled fire or release of a toxic substance, clear the laboratory IMMEDIATELY and, if necessary, adjoining laboratories. Close the laboratory doors. Re-entry into the affected area may be made ONLY IF: ● Any fire is localised, and there is no danger of flash-over if the laboratory door is opened. ● The means for dealing with the problem, and for effecting decontamination, have been properly decided. AND IF 13 ● Breathing apparatus is worn and the training protocols are strictly followed. DO NOT TAKE RISKS. When in the affected area, improve ventilation FIRST by opening windows and turning on the fume hood draught. ● In addition to the breathing apparatus a laboratory coat and any other necessary protection (e.g. rubber gloves) are worn. ● A SECOND PERSON, similarly attired is available to act as a back-up immediately OUTSIDE the affected area. This second person must have the person entering the area in full view so that immediate assistance could be given if required. ● NO RISKS ARE TAKEN. IF IN DOUBT LEAVE MATTERS TO THE PROFESSIONAL FIRE SERVICES. If a fire is not involved, but re-entry is made to deal with a toxic hazard, close the laboratory door after opening the windows and ensuring that the fume hood fans are switched on BEFORE dealing with the problem. DAVID CHAMBERS-ASMAN GAMBLE OR DANE TOPLIS MUST BE INFORMED IF THE BREATHING APPARATUS IS USED SO THAT THEY MAY GET THE AIR SUPPLY RECHARGED. 5. Basic First Aid These notes are intended only for the initial treatment of injuries; in all cases seek medical attention immediately – see inside back cover for emergency telephone numbers. The names of current First Aiders are printed on the lists attached to the breathing apparatus cabinets on the rear stairs opposite laboratory C33. Always: ● ● be calm; give confidence to the injured person. ensure that there is no further danger to the casualty or yourself. (a) Burns ● The effects of burns and scalds are similar, and seriousness depends on area and extent rather than the depth of injury. All burns are complicated by shock and subsequent infection. 14 ● The area of most burns and scalds, including the clothing involved, is usually sterile initially and every effort should be made to keep it sterile. ● HEAT BURNS: Serious heat burns should have a dry sterile dressing applied. Do not use an adhesive wound dressing or ointment of any sort. ● Clothing etc. sticking to burns should not be removed. Never burst blisters. ● SMALL HEAT BURNS: Immerse in clean cold water for a minimum of 20 min. Apply a dry sterile dressing to the area concerned. ● CHEMICAL BURNS: Flush with cold running water for about 20 min. and apply a dry sterile dressing. ● COLD BURNS: These may be caused by cryogenic materials such as solid CO2 or liquid N2. Thaw slowly in lukewarm water and then cover with a sterile dressing. Medical help must be sought if the area frozen is extensive or deep. Do not remove adhering clothing until thawed thoroughly. (b) Chemical Splashes All corrosive acids and alkalies should be washed off the skin immediately with plenty of water if necessary. Cover any wound with a dry dressing. ● There may be a more appropriate action in the case of specific chemical reagents (see the appropriate wall charts). In the case of bromine, for example, wash off the skin with light petroleum, followed by water. If in doubt, wash off only with water; some organic solvents have the ability to carry chemicals through the skin. ● EYES: Chemical splashes in the eye must always be washed out immediately with water. Use an eye wash bottle filled freshly with cold tap water; if one is not immediately to hand, then use the emergency shower at a low flow rate after flushing out the pipework. Medical help must be sought. (c) Cuts The way to control blood loss is to restrict the flow to the wound and therefore encourage clotting. This can be done by pressure or elevation. ● Apply pressure through a suitable sterile pad with thumb and fingers to close the sides of the wound together. Avoid contact with the casualty's blood. Raise the injured part if possible and 15 apply a sterile bandage. This should be tight enough to control bleeding but not so tight as to cut off circulation. If stitching is required, escort the injured party to the Cripps Health Centre or Queen's Medical Centre. If more serious, continue with the first aid until the ambulance arrives. (d) Minor Injuries Suitably stocked First Aid boxes are positioned throughout the building. Stocking is controlled by the Department; report any deficiencies to Mr. A. Spibey so that the supplies may be replenished. For treatment refer, if necessary, to the appropriate wall chart; see also the bibliography section at the end of this manual. (e) Poisoning ● Quickly ask the conscious patient what has happened in case he loses consciousness. There may be a specific antidote. Keep any vomited material for examination, BUT DO NOT INDUCE VOMITING. See that medical help is summoned immediately. ● Meanwhile, if the lips show signs of burning, cool them by giving the casualty water or milk to sip. ● Call a doctor or an ambulance. Try to identify what the casualty has swallowed, and tell the doctor or ambulance control officer. ● If unconsciousness occurs, but normal breathing continues, lay the person in the recovery position as detailed below. TO LEAVE AN UNCONSCIOUS PATIENT LYING ON HIS BACK MAY CAUSE THEIR DEATH. If breathing stops, resuscitation (CPR) becomes necessary – this should only be attempted if you have received First Aider training. Resuscitation of a casualty of poisoning requires special techniques and facilities. (f) Unconscious Patients ● Lay the person in the recovery position (shown below), make sure that breathing continues and that his air passages are not blocked. Ensure that medical help (and a First Aider) has been summoned. 16 (g) Electric Shock Even 60 volts AC may be sufficient to cause unconsciousness and death. ALWAYS SWITCH OFF CURRENT FIRST BEFORE APPROACHING THE PATIENT. Summon medical help. If breathing has stopped, summon the nearest First Aider to start emergency CPR. 6. Accident Reporting The HSE have to be notified about some types of accident Ð hence ensure that all relevant details are recorded at the time. An Accident Report Form (available online: http://uiwwwliv01.nottingham.ac.uk/ARI/) must be completed for all accidents involving: ● ● ● ● ● ● ● ● Personal injury (even if only slight).** Explosion. Fire necessitating the use of any item of fire-fighting equipment. Any accident necessitating the use of the breathing apparatus. Spillage of toxic or obnoxious material necessitating the evacuation of the laboratory. Spillage of highly toxic material, even if safely contained by the fumehood. Failure of apparatus or equipment if it could have resulted in personal injury or damage. Failure of services that could have resulted in a serious accident. ** Please note: By far and away the most common sources of injury arise from cuts and from the results of chemical action on the skin. Cracked and chipped items of glassware should be given to the glassblower for repair. Protect your hands when attaching PVC or rubber tubing to condensers, etc. Wear rubber gloves if there is the slightest risk that chemicals might contact your skin; wear heavy gloves, or use clamps, when handling hot apparatus. 17 E. RULES GOVERNING THE USE OF THE CHEMISTRY BUILDING 1. Normal Working Hours The Chemistry Department is open, and staff are usually present from 08.30 to 18.00 hours, Monday to Friday, excluding official University holidays that indicate the buildings are shut. This constitutes 'normal working hours'. Out of normal working hours you must sign in and out in the book kept in the corridor between the main foyer and the workshop block. When the building is locked, entry and exit is available to official card-key holders only. Cardkeys are to new researchers when they start, and may not be lent out. Anyone admitted by a cardkey holder is deemed to be entirely the responsibility of the card-key holder. In an emergency, other exit doors can be opened with the main door key. If accidently locked-in by the Security Staff, telephone the Security Control Office (Internal Tel. No. 13013). 2. Outside of Normal Working Hours Operations outside of normal working hours are subject to the following conditions: ● Experimental work may be performed only if there is at least one other research worker within easy calling distance. No radios etc. may be played! ● Experimental work is restricted to operations with which the individual is familiar and which are not especially hazardous. ● You are responsible for ensuring that all services not required are turned off on departure. Windows and doors must be shut, you must sign-out in the ‘Out-of-Hours’ register, then lock the outside door on departure. ● Any experiment left running is subject to the usual overnight card regulations (see Section E 4). 3. Building Services Any failure or fault in the gas, water, electricity, or drainage services, or in ventilation (including fume hood draught) should be reported to Mr. D. Chambers-Asman (C6) or Mr. D. Toplis (C2). ● Services MUST be turned off when they are no longer required. Particular care must be taken with connections to the water supply and drainage from apparatus – floods can be 18 exceedingly expensive and create unnecessary animosity with the other sections of the Department. ● It is particularly important to switch off electrical apparatus – including fume hood fans – if no longer required. Occasionally, the electricity supply to the Department suffers interruptions. On reconnection, the sudden surge to equipment left on can result in blown fuses or the tripping of safety devices. Thus vital equipment and important experiments can be jeopardised by laziness or lack of thought. 4. Overnight or Unattended Experiments. Experiments that are left running overnight, or without close supervision, are a constant source of potential disaster for, should anything go wrong, then immediate remedial action would obviously not be available. Overnight or unattended experiments are allowed only if the following protocol is strictly obeyed: ● The water supply must be connected by flexible plastic tubing. ● All flexible tubing must be firmly clipped onto the apparatus, the supply tap, and onto any intermediate unions. The simple sleeve fitting of one flexible tube inside a flexible tube of larger diameter is insecure, and is not allowed. ● The experiment must be in a stable condition when it is left (e.g. boil up rate in reflux must have been steady for the preceding half hour). Insecure joints (stoppered) must be secured with spring clips. ● The area immediately surrounding the equipment must be cleared of chemicals (especially inflammable solvents) and any other equipment or debris not in use. ● The experiment must be accompanied by a properly completed 'Overnight Card'. Write down on the card the information that a Security Officer (a non-chemist) would need to deal with any mishap. Use full chemical names only. ● Heating must be by electrical means only. ● 'High risk' experiments, including those involving > 500 ml of inflammable solvents, must be checked by an experienced research worker (i.e. your supervisor or his nominee) who must countersign your overnight card. 19 F. SAFETY IN THE LABORATORIES 1. Good Chemical Laboratory Practice In all of your work in these laboratories you must adopt good chemical laboratory practice (GCLP) to safeguard both your health and safety and that of others who may be affected by what you do, or fail to do. Basically, this means: a) Safety glasses must be worn at all times within the chemical laboratories. A laboratory coat, properly fastened, must be worn at all times in the labs. Do not expose large areas of skin to possible chemical attack (i.e. no bare arms, bare legs, open-top shoes). Tie-back long hair. b) Complete your COSHH assessment and have it checked, before you start work on any experiment. If you are unsure of the properties of any substance, then adopt the minimum protocol of GCLP + fumehood + rubber gloves. c) Overnight and unattended experiments must be accompanied by an ‘Overnight Card’. The card must detail sufficient information so that a non-chemist can deal with any mishap safely in your absence. Note: if you abuse this system, this concession will be withdrawn! d) Clear-up all spillages immediately. Keep your bench, fumehood and communal areas clean and tidy at all times. Dispose of chemical waste (by the approved method for that material) as soon as it is obtained – not at a later date. Wash-up apparatus at least on a daily basis. e) Only water-soluble non-toxic and non-obnoxious chemicals may be disposed of via the sinks (e.g. acetone rinsings of reaction flasks are OK, dimethyl sulfoxide residues are not OK). Other waste must be place in the approved bins (glass in the glass bin, silica or alumina in the silica bin, paper towels in the general waste bins, syringe needles in the sharps container, etc.). f) Write-up you lab. notes as the experiment progresses – not later. Keep all information, spectra etc., generated by the experiment in good condition, and make sure that it is all crossreferenced to that experiment. g) All chemical samples must be properly and securely labelled (felt tip on glass is not acceptable!), and stored safely. All reagents used in the experiment must be returned to their usual locations as soon as possible. 20 2. Additional Safety Measures Various items for the additional protection of the body are freely available in the Department, and must be used as demanded by the nature of the work in hand. Specifically:● Pull down the front of a fume hood when the experiment therein is underway and requires no further immediate attention. ● Use the safety screens provided for all experiments that are thought to be hazardous. Reactions conducted in Carius tubes must be further shielded by purpose-made metal screens. ● Refuse entry into the laboratory by anyone who is not wearing eye protection. ● When necessary wear suitable gloves; rubber or plastic to avoid chemical contamination of the hands; heavy fabric for handling hot apparatus or sealed tubes. Never immerse gloved hands into chemicals for rubber and plastic are permeable to many compounds. Wear double gloves when handling Hydrogen Fluoride or its solutions (outer glove to be of PVC). ● Wear a dust mask when handling light powdery materials, or when using a mechanical grinder. 3. Preventing Accidents (a) Fires Almost all organic solvents are flammable. The only common exceptions are CCl 4, CHCl3 and Cl3C.CH3 (inhibisol). Many solvents are highly flammable since they have high vapour pressures and low flash points. HENCE: ● Smoking in the laboratories is strictly prohibited. ● Check nearby experiments before lighting a Bunsen burner. ● Apparatus design must prevent solvent vapours from contacting ignition sources (even a hot plate or mantle). ● Solvent stills (if allowed by the Safety Officer) must be sited in safe areas, away from escape routes. Insecure joints must be fitted with spring clips; glass taps must not be capable of falling out. 21 ● The doors to the solvents cabinets must be kept firmly closed. Keep fire doors closed at all times. ● No more that 50 litres in total of flammable solvents (including waste solvents) may be housed in any one laboratory. ● Solvents containers must not be left alongside operational experiments. ● DO NOT store solvents in open containers in the refrigerator. ● Always use anti-bumping agents or rapid stirring before boiling liquids. If you forget you must allow the liquid to COOL FIRST, UNDISTURBED, before adding the nucleating agent. ● The overnight room must be used for high fire-risk experiments. ● Finely divided hydrogenation catalysts must be wetted before disposal; noble metals should be saved for later recovery. ● The alkali metals, their hydrides and organometallic compounds and complex hydrides can inflame in air or on contact with other reagents. Ensure that you receive proper training in their use and disposal from your supervisor. NOTE: Separate regulations apply for the use of: ● Air Sensitive Pyrophoric Reagents. ● Photochemical Reactions. The appropriate specialist Safety Booklets are kept in the box of Safety Manuals in each laboratory; they must be read before such work is started for the first time. Such experiments are classified as Category A (direct supervision essential) until you have been trained in the necessary procedures. (b) Explosion An explosion can arise from a distinct detonation (even in an open vessel), or from the build-up of excess pressure, or from an uncontrolled exothermic reaction. Severity is linked to scale, but some compounds detonate with extreme violence even in small quantities. For example, 5g of acetyl peroxide destroyed the hands of a chemist handling the neat material. Potential Detonators include: ● Organic perchlorates. perchlorates. ● Diacyl peroxides RCO.OO.COR (R = alkyl). ● Most diaroyl peroxides (ArCO.OO.COPh) and polynitro-aromatics must be stored WET to prevent their accidental detonation. Some are exceedingly dangerous – always check BEFORE preparing them. Be aware of your chemistry if using perchloric acid or inorganic 22 ● Some peracids (e.g. dinitroperoxybenzoic acid) are highly dangerous. CHECK BEFORE MAKING PERACIDS. Some literature recipes for peracid syntheses are not to be trusted. ● Peroxides can arise through the autoxidation of olefins, ethers, and alkyl aromatics, especially if they contain an tertiary C–H group. NEVER store these materials in bottles with ground glass stoppers. DO NOT DISTIL TO DRYNESS. Test for peroxides before using old reagents in this category. ● Do not prepare diazomethane in scratched or ground-glass apparatus. ● Use AZIDES with great care; do not allow sodium azide to contact lead metal or lead salts, including lead sink troughs. ● SILVER AZIDE is a very sensitive and violent explosive. It can form in solutions of ammoniacal silver nitrate (silvering solutions) if not made up correctly. DO NOT store such solutions – they can DETONATE SPONTANEOUSLY when moved. Warning to others: Potentially explosive chemicals must be labelled "DANGER – POTENTIAL EXPLOSIVE" and the BS 5378 'Caution - Risk of Explosion' sign used. The following mixtures frequently detonate: ● ● fluorine and organic compounds. alkali metals and polyhalogenated compounds. concentrated or fuming nitric acid and organic compounds. nitrogen oxides and organic compounds complex hydrides and alkyl ethers at about 1600C. permanganic acid and organic compounds. strong oxidising agent/reducing agent mixtures. alkylating agents and inorganic perchlorates. concentrated H2O2 solutions (or inorganic peroxides) and several classes of organic ● compounds. iodine and ammonia solutions (NI3 is a highly dangerous detonator). ● ● ● ● ● ● ● NOTE: Separate regulations apply to work involving use of: ● ● Hydrogenation equipment (both atmospheric and high pressure). Concentrated (>30%) hydrogen peroxide solutions (see Prof. Anderson). The appropriate specialist Safety Booklets are kept in the box of Safety Manuals in each laboratory; they must be read BEFORE such work is started for the first time. Such experiments are classified as Category A (direct supervision essential) until you have been trained in the necessary procedures. 23 Some other explosion risks: ● ● ● ● ● Sealing ampoules. Beware of gas liberated in the reaction. Take GREAT CARE not to condense LIQUID OXYGEN when cooling an ampoule prior to sealing it. DO NOT subject acetylenes and other endothermic compounds (e.g. EtO2C.N=N.CO2Et) to pressure or rapid heating. Ensure all air is removed from hydrogenation apparatus BEFORE admitting H2 gas. Take great care AND SEEK ADVICE (also see p. 38) before destroying residues of the alkali metals, metal hydrides and complex hydrides. NEVER add water to concentrated mineral acids; slow inverse addition with stirring and cooling must be employed. (c) Implosion Occurs when apparatus under vacuum suffers a catastrophic structural failure. The effect can be as bad as those resulting from an explosion. Vacuum desiccators must be used with their protective covers in place. Evacuated bulbs must be taped or fitted with purpose-made screens. Carry out vacuum distillations routinely behind safety screens. AN ACCIDENT REPORT FORM (see p. 17) MUST BE COMPLETED FOR ALL EXPLOSIONS, EVEN IF THERE IS NO INJURY OR MATERIAL DAMAGE. 4. Common Causes of Personal Injury The majority of injuries sustained in chemical laboratories are from carelessness, and the failure to take sensible preventative measures. All injuries must be treated immediately (See First Aid, p. 13) and an accident report for completed (see p. 16). A listing of qualified First Aiders in the Department is posted on the breathing apparatus cabinets located on the rear stairs opposite lab. C.33. a) Cuts The main causes for cuts can be eliminated by taking account of the following: ● Do not used chipped or cracked glass apparatus. Take it to the glassblowers for repair if it is repairable. ● Do not leave glass or metal sharps lying around. Syringe needles must be placed in the "Sharpsafe" containers provided. When full these containers should be given to Mr. D. Toplis for incineration. Other glass and metal sharps MUST be placed in suitably designated receptacles (not the general purpose waste bins); when full, empty the sharps container directly 24 into the large refuse bins located in the Chemistry Department car park. Note: Syringes must be rendered unusable before disposal. ● You must use the carriers provided when transporting Winchesters. The Stores have instructions not to supply Winchesters unless the customer has a Winchester carrier. ● Always soften plastic tubing with hot air or hot water before attaching to glass tubing. Lubricate the plastic or rubber tubing with grease or water and PROTECT YOUR HANDS with heavy gloves or a heavy cloth while inserting the glass tubing. Avoid using excessive force. ● Remove plastic or rubber tubing from glass by cutting it off. ● Wear, or use, adequate protection during experimental work. b) Dermatitis This arises from chemical attack of the skin, and may appear as a rash or in the form of more serious lesions. Some chemicals have a sensitising action. Some individuals are much more sensitive to chemicals than others. Seek medical attention at the Cripps Health Centre if you develop dermatitis. KEEP CHEMICALS OFF YOUR SKIN AT ALL TIMES. DO NOT IMMERSE GLOVED HANDS IN CHEMICALS SINCE RUBBER AND PLASTIC ARE PERMEATED BY MANY CHEMICAL COMPOUNDS. Accidental splashes must be washed off the skin immediately. Use cold water unless there is a more appropriate remedy. c) Chemical Burns Many chemicals have a caustic or other severe damaging effect on the skin. Hence, adequate protection must always be worn. PVC gloves are compulsory wear for handling hydrofluoric acid solutions (see also p. 33). IF ANYONE IN YOUR LABORATORY SUFFERS SPLASHING WITH SUCH CHEMICALS, ASSIST THEM IMMEDIATELY TO AN EMERGENCY SHOWER, AND DRENCH THE AFFECTED AREA WITH WATER. Eye injuries SHOULD NOT OCCUR (eye protection!!!). If an injury should occur use either an emergency shower at low pressure or an eyewash bottle freshly filled with cold tap water or freshly opened sterile eye-washing solution. Seek medical attention. 25 d) Heat Burns and Cold Burns Adequate protection must always be worn when handling hot objects or cryogenic materials. Suitable clamps are often good alternatives to heavy gloves for manipulating hot flasks, etc. ● Burns arising from a fire or hot objects must be given basic First Aid (see p. 13), and then medical attention obtained if necessary. ● Burns caused by cryogenic materials such as solid carbon dioxide or liquid nitrogen should be treated promptly (see First Aid, p. 13). Medical attention must be obtained if the area frozen is extensive, or if it is deeply frozen. e) Ingestion NEVER inhale, taste, or swallow ANY chemical. Always use a mechanical filler on pipettes; NEVER pipette liquids by mouth. Wash your hands regularly, and especially before taking food. Eating, drinking and smoking in the laboratory are prohibited. f) Electric Shock All electrical equipment in the Department is tested for electrical safety on a regular basis. Hence, you must not import electrical items into the Department. ● D.I.Y. repairs of electric equipment ARE PROHIBITED. Return items for repair if bare contacts or wires are exposed, or if the cable is frayed. ● If the fuse blows, get the equipment checked. If an electrical trip-switch operates, first check for likely causes (e.g. short circuit) and remedy BEFORE reconnecting the electrical supply. ● Turn off the power supply BEFORE giving First Aid (see p. 16) to anyone suffering from electric shock. Medical attention must be sought for anyone injured by an electric shock. g) Other Injuries Other injuries may occur from time-to-time, especially those of the "domestic variety". Give basic first aid (see p. 14) and obtain medical help if necessary (see inside back cover for emergency telephone numbers). 5. Tidyness Sloppy, untidy work breeds accidents – often for other, innocent people. Hence, clear up all spillages immediately. Clear up all other debris generated by an experiment as soon as possible. Do not allow dirty apparatus to accumulate; wash-up at least on a daily basis. 26 The remnants of old experiments must not be allowed to accumulate. Dispose of chemical waste regularly (see p. 37). Keep work areas as tidy as practicable; paperwork should be stowed away, and coats and bags kept in the cloakrooms provided. DOORWAYS, PASSAGEWAYS AND OTHER ESCAPE ROUTES MUST BE KEPT CLEAR AT ALL TIMES. Securely label all chemical products you make. Felt tip marking on glass is not acceptable. Make sure that all of your personal commercial chemicals are coded with your supervisor's initials, and the month and year of purchase (e.g. RMD/11/09). Future receipts must be documented immediately on arrival and entered onto the COSHH Database. PLEASE NOTE: Routine audit checks are made of all laboratories, and the Safety Personnel have the authority to close down any laboratory considered to be unsafe. Individuals can be suspended from further experimental work. 6. Communal Facilities a) Research Laboratories i) Many of the facilities and much of the equipment provided in the individual research laboratories are for the use only of the personnel assigned to that laboratory. This includes fume hoods, refrigerators, ovens, rotary evaporators, electrical equipment, other instrumentation, vacuum frames, and laboratory general chemicals and solvents. ii) These facilities, apparatus and chemicals may not be used by personnel from other laboratories without the knowledge and permission of the academic supervisor(s) of the personnel in the laboratory containing the apparatus or chemicals. iii) You must adopt a responsible attitude towards the communal facilities in your own laboratory for anti-social behaviour will not be tolerated. In particular you must clean out thoroughly any communal piece of equipment or apparatus after you have used it – especially rotary evaporators. iv) Remove all of your debris from the communal work areas on completion of your experiment. This includes chemical reagents used in, and chemical waste produced by the experiment. v) Use sealed containers, properly labelled, for all chemicals kept in the laboratory including items stored in the refrigerator (or cold room store). 27 vi) Keep clear those areas on your own work bench that have been assigned to communal equipment. Arrange for the replacement or repair of any item that is damaged whilst you are using it. KEEP ALL GANGWAYS AND EXITS CLEAR. vii) Personnel in each laboratory are assigned to specific tasks to ensure efficient and safe working (e.g. waste solvents, solvent stills, fumehoods, etc.). In addition, it is expected that each laboratory shall adopt a clean-up and disposal routine by the last day of every month. A form noting that this self-certification has been completed must be signed by the laboratory’s safety monitor, countersigned by the member of the academic staff in charge, and given to the Organic Safety Officer. Regular inspections will be held, and unsatisfactory laboratories will be closed until improvements are made. b) Instrument Laboratories and other General Facilities A Responsible attitude towards the facilities provided in the communal intrument laboratories is vital for ensuring their continued smooth operation. Make sure that you clear away all of your equipment, apparatus, and debris immediately after completion of the task in hand. Report promptly any malfunction or other problems to the technician in charge of the facility. c) The Hydrogenation Room This room, which is located above C.33, is not available on an open-access basis. The room is locked at all times, and the key for the door is available on request from the Mr. Dane Toplis. The log book should be completed before receiving the key, and the key must be returned to Dane Toplis after each trip to the hydrogenation room. The sign by the stairs leading to the hydrogenation room should be used to indicate whether the room is ‘occupied’ or ‘vacant’ – i.e. move the slide indicator to show ‘occupied’ before entering, and return it to ‘vacant’ when leaving. Regular inspections of the room are made, and any breakage or spill will be attributed to the person who used the room immediately before the inspection. Hence, anyone finding the room or equipment in an unsatisfactory condition before commencing work should report the matter to Dane Toplis. Serious transgressions (e.g. failure to clear-up spillages, to get damaged apparatus repaired, or abuse of the key system) may result in the offender being permanently barred from future use of the facility. d) Refrigerators and Deep Freezers All materials put in refrigerators and deep-freezers must be in air-tight containers and be clearly labelled with the names of both contents and the research worker. Commercial chemicals must be 28 coded (see pp. 10 and 30) on the label. Foodstuffs must not be stored in refrigerators or deepfreezers. Problems should be reported to Mr. D. Toplis. NOTE: Inflammable solvents in open containers pose a very real and important fire and/or explosion risk. Because of this all chemicals refrigerators and freezers must be fitted with external thermostat controls to obviate sparking. Refrigerators and freezers must be defrosted regularly, and certainly before the build-up of ice causes problems of removal of containers, shelves or drawers. G. CHEMICALS AND SAFETY 1. General Considerations All commercial chemicals on receipt must be coded on the label (use a permanent felt tip marker or heavy pencil) with your supervisor's initials, and the month and year of purchase (e.g. GP/11/98). The appropriate entry MUST also be made onto the COSHH Database. All compounds made during the course of your work must be properly labelled with the name or formula, the date, your name, and any hazardous properties (e.g. 'HIGHLY TOXIC'). The use of felt tip on glass for labelling purposes is not acceptable. Chemicals must be stored in the purpose-made cabinets, chemical storage rooms or cold stores and not left out on bench tops, on the floor, by sinks or in fume cupboards. The storage should be as follows: ● ● ● ● ● Solvents are kept in the solvents cabinets. Chemicals which must be stored below 40C are kept in a cold store. Other chemicals which have a CORROSIVE, IRRITATING, HARMFUL or TOXIC attribute are kept in the vented cabinets beneath the fume cupboards. Relatively innocuous chemicals may be stored in the room storage (if provided) Be careful not to store powerful oxidising agents or pyrophoric materials amongst readily combustible chemicals! Empty chemicals containers MUST be thoroughly washed-out BEFORE disposal, and the labels and caps removed. They should be taken to the skip EACH day. 29 2. Chemical Toxicity Levels of toxicity of known compounds can be found in the books available in C.30, the Chemistry Library, and the Science Library. There are many indices of toxicity, but the two you may meet most commonly are: LD50 (Lethal Dose 50) – a calculated dose of a substance which is expected to cause the death of 50% of an entire defined experimental population. Occupational Exposure Limit – refers to the maximum air concentration of a chemical to which an individual can repeatedly be exposed 8 hours/day, 5 days/weeks. It is to be stressed that individuals react to chemicals to different extents, with some people being allergic to chemicals which produce no ill effects in others. Some chemicals have a sensitizing action. If you find yourself especially sensitive to a chemical, discuss the problem with your supervisor. a) Highly Hazardous Chemicals Hazards are, of course, noted with each and every chemical listed in the COSHH Database together with recommendations for their safe handling. In several cases the ACCIDENTS button in the Database will alert you to potentially incompatable chemicals, or to problems that have arisen through use of specific chemicals. However, you must accept that the Database entries can never be completely comprehensive, and you should always proceed with caution. Compounds signified * below may not be stored in research laboratories, and may be obtained from the Main Chemical Store only against the signature of the academic supervisor of the project. Listed below are particular classes of compounds which generally have high toxicity: ALKYLATING AGENTS - HIGHLY TOXIC, CARCINOGENIC Includes: alkyl halides, tosylates, mesylates, trifluoromethane sulphonates. dimethyl- and diethyl sulphate (and other alkyl sulphates). methyl fluorosulphonate* acrylonitrile, acrolein, crotonaldehyde diazomethane (and diazoaliphatics). vinyl chloride and bromide chloroform, and carbon tetrachloride. formaldehyde, ethylene oxide, b-propiolactone, nitrogen mustards. chloromethyl methyl ether and bis(chloromethyl) ether. possibly other 30 INORGANIC TOXINS AND ORGANOMETALLIC TOXINS Includes: cyanide salts,* hydrogen cyanide,* hydrogen sulphide,* carbon monoxide,* nickel tetracarbonyl,* phosgene and thiophosgene. nitrogen oxides hydrazines and their salts (toxic and/or carcinogenic; sensitizers) osmium tetroxide (keep a solution of asorbic acid or “Mazola” cooking oil to hand in case of spillage). heavy metal salts. selenium dioxide and elemental selenium. nickel, chromium, beryllium, cadmium and arsenic compounds. Bear in mind that: – certain organic compounds may decompose to release such toxins (e.g. cyanohydrins decompose to give HCN). – organomercury, organoselenium and organothallium compounds and other derivatives of heavy metals can be at least as toxic as the inorganic salts of these metals, and are frequently much more toxic. SOLVENTS - TOXIC AND CARCINOGENIC Includes: chloroform carbon tetrachloride benzene hexamethylphosphoramide 1,4-dioxane Avoid these solvents if at all possible. Dichloromethane may frequently be substituted for chloroform or carbon tetrachloride. Substituted ureas such as tetramethylurea can sometimes be a good replacement for hexamethylphosphoramide. Toluene is often a satisfactory replacement for benzene. If the listed solvents must be used, then the use of a fume hood is obligatory for all operations involving that solvent. OTHER TOXIC AND/OR CARCINOGENIC COMPOUNDS Many compounds besides those mentioned below may pose a toxic hazard. Includes: isoamyl nitrite N-nitroso compounds most aromatic amines polycyclic aromatics and heterocyclics intercalating compounds ethidium bromide 31 acetamide fluoroacetic acid and derivatives urethane 1,2-dialkylhydrazines diazoalkanes, azoalkanes, azoxyalkanes guanidines NOTE: The Carcinogenic Substances Regulations 1967 PROHIBIT the use or importation of 2naphthylamine, benzidine, 4-aminobiphenyl and 4-nitrobiphenyl. Controls were also imposed on the use of 1-naphthylamine, o-tolidine, dianisidine, dichlorobenzidine, auramine and magenta. Among naturally occurring carcinogens, mention should be made of the potent tumour-promoting properties of the phorbol esters (from croton oil) and the aflatoxins, especially aflatoxin B1 (from aspergillus flavus). Several chemical compounds may not be purchased, isolated from natural sources, or synthesised without Home Office permission under the Misuse of Drugs Regulations. POWERFULLY CORROSIVE MATERIALS – INCLUDING HF SOLUTIONS Since these materials rapidly destroy skin tissue, you MUST wear the appropriate gloves at all times whilst handling them. Contact with solutions of HF is particularly insidious as HF has an anaesthetizing action on sub-cutaneous tissue. HF burns can be very deep-seated and difficult to cure. Anyone contemplating using HF must obtain calcium gluconate HF burn cream before commencing experimental work. You must wear double gloves when handling HF solutions, the outer gloves being of PVC (or the material currently recommended for HF). b) Mercury Metal The TLV for mercury is 0.05 mg/m3 whereas air saturated with mercury vapour at 250C contains about 10 mg/m3. At 500C the concentration is about 10 times greater. Poisoning by mercury vapour is slow, insidious, cumulative and often unrecognised before irreversible damage is done. This means that poorly ventilated rooms should never contain exposed mercury surfaces, and even with good ventilation the exposure of mercury to air must be kept to the minimum. Exposure most often arises from spilt mercury; the liquid globules run into cracks and crevices and can remain an unnoticed source of vapour for a long time. Do not transfer mercury from one vessel to another EXCEPT over a tray or basin (glass, plastic, iron or stainless steel) that will catch any spillage. Keep mercury containers closed at all times if you can, but if a mercury surface has to be at atmospheric pressure (as in some manometers and reservoirs), limit the diffusion of the vapour by covering with a layer of water and by use of cotton wool plugs. Manometers should be designed with a stopcock at the vent which can be closed when the apparatus is not in use. 32 Treat spillages as follows:● contain the spillage to as small an area as possible ● IDEALLY the droplets or pool of mercury should be collected using a fine capillary connected to a filter flask and water pump. ALTERNATIVELY, scoop up as much of the mercury as you can into a glass, plastic or iron container. THEN make up a little scoop of paper and use a small brush or wad of tissue to help the droplets onto the paper. surfaces affected by tiny droplets of mercury in a spillage should be decontaminated by pasting with a slurry of slaked lime and flowers of sulpur mixed with a little water.* The slurry should ● be allowed to dry and about 24h later it should be removed with clean water and the surfaces again allowed to dry. Mercury is converted into its involatile sulphide. * This mixture is somewhat caustic. Slaked lime is Ca(OH)2, and is obtained by mixing CaO with about 1/3 of its weight of water. Mercury vapour in the atmosphere can be monitored. See Mr J. Gamble for this facility if you have reason to believe that your laboratory is contaminated. NOTE: Many mercury compounds are toxic by ingestion, inhalation, or through skin absorption. Mercuric salts tend to be lipid soluble and can permeate rubber or plastic gloves. Handle only in the fume hood and treat spillages as for mercury metal. Wash off the hands with water, or water and soap for water-insoluble compounds. c) Obnoxious Chemicals Most sulphur(II) and some sulphur(IV) compounds are stench chemicals, even at very low concentrations. The same problem arises with some compounds of selenium and phosphorus. These materials MAY ONLY be handled in an efficient fume hood. They MUST NOT be poured down the drains. Contaminated apparatus MUST be steeped in an oxidising solution (e.g. sodium hypochlorite). The hazards board outside the laboratory MUST be kept scrupulously up-to-date while obnoxious compounds are in use. 3. Laboratory Hazards Boards A listing of all chemicals in use in the laboratory’s fume cupboards must be written on the board by the laboratory door, and the listing MUST be kept up to date. This is done in black ink. It is particularly important to highlight the use (or disposal) of STENCH chemicals for the reason that the source of the problem can be readily identified if complaints are received from other people 33 inside or outside the Chemistry Department. This is done in red ink only. At low levels, sulphur compounds can be mistaken for a gas leak! 4. Gas Cylinders Cylinders MUST NOT BE LEFT FREE-STANDING – rather they must be kept in a trolley or stand, or chained to a wall or bench. When not in use the gas must be turned off at the main valve, and the reduction valve bled via the needle valve. Where cylinders are issued with operating instructions, these must not be removed. IT CAN BE DANGEROUS TO MOVE A CYLINDER WITH THE GAUGE STILL ATTACHED, FOR IF IT SHOULD FALL OVER THE GAUGE MAY SHEAR OFF RELEASING THE FULL PRESSURE WITH EXPLOSIVE FORCE. GAUGES MUST BE REMOVED BEFORE TRANSPORTING CYLINDERS. CYLINDERS MAY ONLY BE MOVED ON A TROLLEY OR SKATE. Cylinders which have developed stiff, locked, or leaking valves should be returned immediately to the store with a note of the fault attached. The use of liquid ammonia (Category A) is the subject of a separate Safety Protocol which must be read BEFORE training in its use is given by an experienced person. You may use the cylinder of HCl gas only after receiving instruction and training from Mr D. Toplis. It is vital that a rigid protocol is observed with this highly corrosive gas for it is very easy to do irrepairable damage to the reduction valve assembly. The use of cylinders of CARBON MONOXIDE, HYDROGEN CYANIDE, HYDROGEN SULPHIDE, PHOSGENE AND OTHER HIGHLY TOXIC COMPOUNDS REQUIRES SPECIAL PERMISSION AND THE PROVISION OF SPECIAL FACILITIES. supervisor. See your It is expected that lecture bottles of gas, and other small cylinders will be used ONLY in a fume hood, and that: ● the cylinder is securely but loosely clamped to an adequate stand. ● you have received proper instruction in the use of the gas or liquid contents from your supervisor. In all cases where a TOXIC GAS IS BUBBLED THROUGH A REACTION MIXTURE you MUST make adequate provision for absorbing the surplus reagent at the apparatus vent. If in 34 doubt, check with your supervisor how to do this. HYDROGEN AND CARBON MONOXIDE may be vented ONLY IN THE OPEN AIR OUTSIDE THE DEPARTMENT. Take special care with flammable gases. 5. Liquid Nitrogen The main hazards arising from the use of liquefied nitrogen are: ● Thermal shock on the rapid cooling of glassware; an implosion may result if under vacuum. Dewar flasks often disintegrate in this fashion and should always be encased in a rigid container, preferably of metal. Transport Dewars ONLY in the carriers provided. Always wear safety glasses. ● Cold burns resulting from skin contact. Cold buns can cause severe, deep-seated injury. ALWAYS keep liquid N2 off the skin; USE HEAVY GLOVES when handling very cold apparatus. See p. 15 for the treatment of cold burns. ● The inadvertent condensation of oxygen, argon, or other gaseous material. VESSELS COOLED IN LIQUID N2 SHOULD NOT BE OPEN DIRECTLY TO THE ATMOSPHERE FOR LONGER THAN 1 MIN OR SO FOR LIQUID O2 MAY CONDENSE.* Apparatus that is being flushed with argon or carbon dioxide at –1960C WILL ACCUMULATE liquid Ar or solid CO2. ● VESSELS CONTAINING LIQUEFIED O2, Ar, or SOLIDIFIED CO2 AND THEN SEALED WILL EXPLODE WITH GREAT VIOLENCE IF ALLOWED TO WARM UP. TAKE GREAT CARE IN THE COOLING AND SEALING OF AMPOULES. ● Cold traps on vacuum lines must be allowed to thaw (by removal of the liquid N2 Dewar) immediately after the vacuum is released and the trap is reconnected to the atmosphere. * Mixtures of liquid O2 and organic compounds are highly dangerous through explosion and combustion. Liquid O2 will immediately inflame cotton material (e.g. a lab. coat) on contact, and combustion will proceed with great vigour. Liquid N2 is colourless, whereas paramagnetic liquid O2 is pale blue. Care should be taken not to allow the oxygen content of liquid N2 in Dewar vessels to rise to dangerous levels. 35 6. Detoxification and Disposal of Chemicals a) Waste solvents Laboratories are equipped with two large waste solvent containers – one for Non-Chlorinated Waste Solvent and one for Chlorinated Waste Solvent. Waste solvent must be placed in the appropriate container. Chlorinated solvents must not be placed in the Non-Chlorinated Waste Solvent container. The containers must be stored in the vented cabinets beneath the fume cupboards. When these containers are near-full, make arrangements with Mr. D. Toplis for the transfer of their contents to the larger metal storage drums in the fire-proofed laboratory (C.43); these are used for transport by the waste chemicals contractor. Note: Waste solvents must have been though a vaporization-condensation cycle (i.e. distillation or rotary evaporation) before being poured into the appropriate waste solvent container, otherwise the metal drums can corrode severely during the storage period. Mr. Toplis may refuse to accept a batch of waste solvent if it is seen to be contaminated. b) Reaction Residues Modest quantities of water-miscible non-toxic waste (e.g. inorganics, acetone, lower alcohols, reaction residues in acetone, etc.) may be flushed down a drain with plenty of water. DO NOT dispose of DMSO in this way since it usually contains Me2S (STENCH!!!). Small quantities of amines should be dissolved in dilute HCl before flushing down the drain. In general, you should avoid pouring appreciable quantities of chemicals down sinks. You must take positive steps to prevent toxic chemicals from venting into the laboratory or the fume hood, or from contaminating the drains. c) General Disposal Procedures Disposal procedures are given with each chemical listed on the COSHH Database. The book Destruction of Hazardous Chemicals in the Laboratory by G. Lunn and E.B. Sansone (a copy is kept in room C.30) contains a considerable amount of useful information. i) Heavy metal residues, etc. (e.g. Hg, Cr, Pb, Os, Sm, etc.) should be collected separately in suitable containers of 1 litre capacity or less. When the container is full, or no more waste is being collected, submit the material to the Departmental Waste Disposal procedure (see p. 37). Other toxic solids which cannot be chemically detoxified (i.e. notably Se and SeO2) may be disposed of similarly. Note: Only Macintosh-labelled containers are allowed. Heavy metal salts present in dilute aqueous solution, clearly, should be recovered in solid form – otherwise the Department will have to pay a high for the disposal of water! The metal ions should be converted into an insoluble derivative (e.g. Ba2+ → BaSO4, Pb2+ → PbS, Hg2+ → HgS, or 36 [HgI4][Cuen]2 etc., etc.) which is separated and submitted to the Departmental Waste Disposal procedure (see p. 39). Most problems in this category may be solved by consulting a textbook on qualitative inorganic analysis; this is your or your Supervisor’s job – the Safety Officers will not do your literature work for you. ii) Sulphur stench compounds Sulphur(II) and sulphur(IV) compounds and their reaction residues must be digested in sodium hypochlorite solution. When the stench has been removed, the hypochlorite solution may be poured down the fumehood sink with plenty of water. If, however, the stench is not removed in, say, 2 weeks arrange for disposal through the Departmental Waste Disposal Service. iii) Cyanide residues must be digested in sodium hypochlorite solution for > 1 week before disposal down a fumehood drain with plenty of water. iv) Chromatography column absorbents (e.g. silica gel and alumina) must be placed in a plastic bag which is then placed in the special dustbins provided. v) Noble metals and their residues (notably Ru, Rh, Pd, Ir and Pt) should be collected separately in suitably labelled jars for later recovery. A one-off 50 mg sample is not worth worrying about, but regular use of, say, Pd-C should lead to saving the residues. These should be stored under water since the dry reagents may be pyrophoric. vi) Chemically reactive substances Many organic and inorganic compounds can be deactivated and/or detoxified by a suitable chemical reaction. Hydrolysis may be suitable in many cases (e.g. small quantities of acid chlorides and anhydrides, hydrolysable 'metal' chlorides such as POCl3, AlCl3, SnCl4 etc., HMPA under acid catalysis as noted below). It is difficult to be specific because of the great variety of compounds in use. Always check the proposed disposal method carefully (you should know the chemical properties of the reagents you handle!) and consult your supervisor and Lunn and Sansone’s book on the Destruction of Hazardous Chemicals in the Laboratory. Do not, for example, destroy residual alkali metals, alkali metal hydrides (MH), organometallics (MR), or complex hydrides with water (explosion risk). Usually a higher alcohol should be employed; see your supervisor first. If such deactivation also leads to detoxification giving water-soluble material, then these residues may be flushed down a fume cupboard drain with plenty of water. If the deactivation does not result in detoxification [e.g SnCl4 → Sn4+(aq.)] then the toxic problem should be handled as noted above. Deactivation, when feasible, is always preferred for the risks posed by the waste in storage, 37 transport, or final land burial is greatly reduced. Contractors may refuse to take items which are in a dangerous state (e.g. bottle of half-used tert-BuLi). Many other organic or inorganic compounds are very readily deactivated and/or detoxified, even when in the vapour state. Again, if you know the chemistry, then you should be able to work-out (or look up) how! A few typical countermeasures are: Hazard Br2 aq. Countermeasure NaHSO3 Hazard O3 Countermeasure aq. NaI HCl gas H2O M(CO)n Br2/H2O If you have a disposal problem that you are unable to solve, then consult your supervisor and Lunn and Sansone’s book on the Destruction of Hazardous Chemicals in the Laboratory. vii) Alkylating agents, urethane, HMPA, etc. Details for the safe disposal of these materials are given by the COSHH Database and Lunn and Sansone’s book on the Destruction of Hazardous Chemicals in the Laboratory (C.30). (d) Departmental Waste Disposal Service This is for all other toxic waste materials which cannot be detoxified further for disposal down a fume hood drain. This category may also contain commercial chemicals which have decomposed, or are no longer required. All of this material must not be in a potentially dangerous state. Make sure the bottles containing the material are properly labelled with the nature of the waste. If any material is particularly toxic, the bottle should be enclosed in a sealed plastic bag. The containers must then be securely packed in a suitable cardboard box such that the risk of breakage during transportation is minimised. Fill in the appropriate documentation (a specimen form is shown on the next page), get this signed and checked by your supervisor and give the form to Mr. D. Toplis; he will make arrangements to remove your waste to store. Spare forms are available from Mr. D. Toplis. It is permissible to mix COMPATABLE chemicals (e.g. carboxylic acids together, or amines together) and this results in substantial cost savings (see the COSHH database for further guidance). The label must indicate the nature of the mixture and hazards. 38 SWS DUTY OF CARE WASTE SPECIFICATION SPECIALISED WASTE SERVICES Environmental Consultants • Waste Management Contractors Waste Producer ............................................................................... SWS Office Use Only Department ...................................................... Sheet No. ............ Information compiled by ................................................................. Control No: ............................. Position .............................................................. Date .................... Date Rec'd: ............................. BOX CODE MATERIAL CONTAINER No. OF HAZARDS SIZE CONTAINERS (e.g. Flamm., Toxic etc.) 39 H. BIBLIOGRAPHY 1. Safety Statement, University of Nottingham, Department of Chemistry (all personnel should have received a copy and/or have access to a copy on the School website). 2. Safety Handbook, University of Nottingham (all personnel should have received a copy and/or have access to a copy on the University website). 3. R.E. Lenga (ed.): The Sigma-Aldrich Library of Chemical Safety Data (1986). 4. N. Irving Sax: Dangerous Properties of Industrial Materials, 6th Edn. Van Nostrand-Reinhold (1984). 5. a b c d Hazard Data Sheets, BDH (1989) and May & Baker (1989). Bretherick’s Handbook of Reactive Chemical Hazards (4th Edition). Benzene: Uses, Toxic Effects and Substitutes; Occup. Safety & Health Series. Occupational Exposure Limits; (1991) H.S.E. e Control of Carcinogenic Substances; (1989) H.S.C. f Destruction of Hazardous Chemicals in the Laboratory, G. Lunn and E.B. Sansone. 6. a b c d e 7. W. Braker and A.L. Mossman: Effects of Exposure to Toxic Gases. First Aid and Medical Treatment, Matheson Gas Products (1970). H. Proctor and P.S. London: Principles of First Aid for the Injured, 3rd Edn., Butterworths (1977). Protection Against Ultraviolet Radiation in the Workplace, National Radiological Protection Board (1978). Safety in Laboratories, Ciba-Geigy (1984). Safety in Universities. Notes of Guidance, The Committee of V.C.'s and P.'s. Safety publications in the Science Library: See listing in ref. 2. 40 I. EMERGENCY TELEPHONE NUMBERS 75 Cripps Health Centre 8888* Emergency Service for Fire and Ambulance 13013 Trent Building Security (24 h cover) – use after 5pm when Emergency Service is needed. * Inform the person who answers the call what service is required, state the location in the Chemistry Building. Give your name and remain available to direct the emergency service responding to the zone concerned. Ensure that someone is stationed outside the Department, in the car park with a view of the service road, to direct the emergency service into the building. OTHER TELEPHONE NUMBERS FOR USE IN AN EMERGENCY LABORATORY SUPERVISOR: See notice on lab door for name and telephone details. ORGANIC Prof. B. Lygo Internal: 13541 LABORATORY MANAGER: Mr. D. Toplis (C2) Internal: 13539 Home: 9198419 DEPARTMENTAL TECHNICAL MANAGER (Plumbing, Electrical And building faults) Mr. D. Chambers-Asman (C6) Internal: 13517 SAFETY OFFICER: UNIVERSITY SECURITY: UNIVERSITY SAFETY OFFICE Internal: 13013 Dr. H.J. Sutherland Internal: 13401 Organic Safety Manual 12th Edition, created 22.09.2014 by Simon Woodward Attended introductory safety talk or Watched video recording □ □ ORGANIC CHEMISTRY RESEARCH GENERAL SAFETY MANUAL I have read and understood the General Safety Manual. I agree to abide by the general protocol, recommendations and prohibitions outlined therein. I further understand that additional protocols and prohibitions may exist for work in specialised areas not covered in this manual, and agree that before work is commenced in these areas it will be thoroughly discussed with my supervisor and the relevant safety manual will be read. NAME (print)............................................... Laboratory.................. SIGNATURE.............................................. Date.......................... SUPERVISOR (print)................................................ SIGNATURE............................................. Date.......................... This form must be duly completed (including the box in the top right) and signed by both student and supervisor, and then given to the Section Safety Officer (Prof. Simon Woodward) BEFORE work is commenced for the first time.
© Copyright 2025