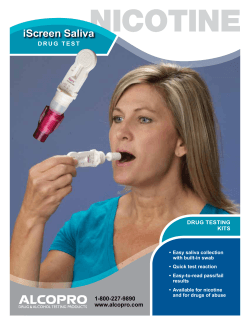
C L N linical
news brief U.S. Hospitals Underperform on Patient Safety Measures An update to the Hospital Safety Score released by The Leapfrog Group revealed that while some hospitals reliably deliver safe healthcare, many still lag in their ability to protect patients from preventable medical errors, injuries, accidents, and infections. Launched in June 2012, the Hospital Safety Score uses 26 measures of publicly available hospital safety data to assign an A, B, C, D, or F score to U.S. hospitals. This new update to the report summarizes data from the past 6 months that was collected from 2,618 hospitals and primarily covers their performance in 2011. On the Hospital Safety Score website, Leapfrog recommends consumers go to hospitals that received an A rating; however, of the Snapshot Hospital Safety Scores Percentage of Hospitals 26% 20 10 0 5% A B C D Assigned Score Will New Clinical Trial Methods Speed Drug, Biomarker Use? 1% F Source: The Leapfrog Group, Hospital Safety Score, 2012 hospitals studied, only 30% earned this top score, with 6% getting a D or F score. These two lowest grades are new additions to the Hospital Safety Score rankings and denote hospitals with the most hazardous environments for patients. Though these numbers are far from optimal, Leapfrog’s findings were not all negative. For instance, hospitals of all classes—teaching, public, and specialty—earned As, with no one type dominating the highest safety scores. Another promising discovery was that not only nationally acclaimed hospitals earned As, but also hospitals serving highly vulnerable, impoverished, and health-challenged populations. Leapfrog also analyzed statewide performance. Hospitals in Massachusetts and Maine topped the list of those garnering As, at 83% and 80%, respectively. With 180,000 patients dying annually from errors and infections, the lack of attention to patient safety in U.S. hospitals remains a grave problem, according to the report’s authors. Through the Hospital Safety Score, Leapfrog aims to help consumers make informed decisions about where to seek care and drive the market toward making patient safety a greater priority. R apid advances in both molecular diagnostics and the understanding of cancer biology are transforming the cancer research and drug development enterprise, which experts say will lead to new clinical trial paradigms and testing protocols aimed at better matching patients to studies and therapies. The oncology community largely is viewing these events with enthusiasm and optimism for their potential to bring cancer drugs to market sooner, improve patient outcomes, and to make real the dream of personalized cancer therapy. Such efforts, which depend heavily on validated biomarkers, also are expected to transform the role of clinical laboratorians in oncology diagnostics and treatment. “We’re at a very interesting point. We’ve now spent the last several decades developing cancer therapeutics, but it’s always been evident that even very successful drugs work in only a fraction of patients and all too often for a limited time. The reasons for that have become much clearer with the introduction in the last fiveto-10 years of new technologies that allow us to characterize in detail cancer biology,” said David Parkinson, MD, a venture partner at New Enterprise Associates, a venture capital firm in Menlo Park, Calif. “We now understand that selection of therapeutic tools ought to match the biological characterization of the patient’s See Cancer Diagnostics, continued on page 2 Molecular Diagnostics Reimbursement in Flux What Will New Codes Mean for Labs? By Bill Malone M olecular testing has become an economic powerhouse for the lab industry and contributes a wealth of valuable information to patient care. But even as researchers and labs look for the latest breakthroughs, the government and other payers are still trying to make up their minds about what these tests are worth and how to keep track of them. This month, the Centers for Medicare and Medicaid Services (CMS) implements a completely new system for coding molecular tests on Medicare claims. For more than a year, the lab community waited in limbo while physician organizations tried to persuade CMS to move the new Common Procedural Terminology (CPT) codes to the physician fee schedule—a change that non-pathologist laboratorians worried would undercut their long-standing professional role overseeing molecular tests. In November, CMS finally decided it would keep the new codes on the clinical lab fee schedule as planned. However, the agency now has replaced last year’s worry for labs with a new one: how will these tests be paid? Rather than carry over the payment associated with the old set of codes labs are accustomed to, CMS asked its local administrative contractors to come up with their own payment schemes from scratch. This so-called gap-filling method has never before been used for such a large swath of the lab fee schedule, and is creating See Molecular Test Coding, continued on page 6 Clinical Laboratory News 30% A New Paradigm for Companion Diagnostics in Cancer The American Association for Clinical Chemistry, Inc. 1850 K Street, NW, Suite 625 Washington, DC 20006 30 January 2013 volume 39, number 1 w w w. a a c c. o rg By Genna Rollins N = 2,618 38% 40 Clinical Laboratory News The authoritative source for the clinical laboratorian in this issue 8 11 Lab 2013 Saliva—A Convenient Diagnostic Fluid 2013 Federal Outlook for Labs Patient Safety Focus Spreading Infections with POCT Devices Failure to Follow Up on Test Results A Physician Talks Lab Error 12 13 15 16 17 18 19 Regulatory Profiles Industry Profiles Diagnostic Profiles News from the FDA @CLN_AACC Greenfield, OH Permit No. 436 paid nonprofit org. u.s. postage Complexity Stymies Drug Development Cancer Diagnostics, continued from page 1 tumor at each particular point in the natural history of the patient’s disease. This means that if we’re going to move toward more efficient cancer therapy we have to have parallel development of biologically targeted therapeutic agents and diagnostics which characterize patients accurately enough for the efficient use of those tools.” Parkinson for 5 years led Nodality, a molecular diagnostics start-up, and he co-chairs the cancer steering committee of the Biomarkers Consortium, a public-private biomedical research partnership managed by the Foundation for the National Institutes of Health. He went on to explain that the old model of single biomarker test results being used to select oncology treatments, monitor the effects of those therapies, and switch therapies as treatment resistance emerges is no longer viable. “It’s becoming clear that in order to be able to predict whether or not a cancer agent is going to work in a particular tumor setting is going to require a much more complex series of measurements, and the opportunity is that those measurements taken in whole can much more accurately predict outcomes related to therapeutics.” K-ASSAY ® Cancer: A Chaotic Brew A substantial body of evidence has dashed forever the notion that cancer is a monolithic disease with a straightforward path to cure. “It now is quite clear that diseases 10 years ago we considered one disease are really a compilation of different tumor types, each with a different driving biology. We now realize that in each tumor type if we just treat with a targeted agent, only a fraction of patients are going to genuinely derive benefit,” said Funda Meric-Bernstam, MD, professor of surgical oncology and medical director of the Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy at the University of Texas MD Anderson Cancer Center in Houston. Oncologists now recognize the disease as a chaotic brew of genetic rearrangements, mutations, deletions, and amplifications. Toss in epigenetic influences on gene expression and evolution under therapeutic pressure, and the recipe for devising effective treatments becomes considerably more complicated, according to John Mendelsohn, MD, co-director of the Khalifa Institute for Personalized Cancer Therapy and former president of MD Anderson. “There are unresolved questions that require aggressive research. The heterogeneity of the cancer. The plasticity of the can- The Assay You Can Trust... Immunoassay Reagents for Chemistry Analyzers™ Suitable for most chemistry analyzers such as Cobas ®, Roche/Hitachi, Beckman Synchron ®, Olympus, etc. Lipid Assessment Apolipoprotein AI Apolipoprotein AII ** Apolipoprotein B Apolipoprotein CII ** Apolipoprotein CIII ** Apolipoprotein E ** Lipoprotein(a) Coagulation Anti-Thrombin III D-Dimer Factor XIII ** FDP ** FDP-E ** Fibrinogen Plasminogen Nutritional Status Ferritin Prealbumin Transferrin Anemia Ferritin Allergy Total IgE Selected List of Cancer TreatmentRelated Companion Diagnostics Inflammation / Cardiac Anti-Streptolysin O Complement C3 & C4 C-Reactive Protein High-Sensitive CRP Wide-Range CRP Ferritin Fibrinogen Microalbumin Rheumatoid Factor CDx 2006 PMA 2012 Cetuximab, Panitumumab Colorectal Cancer BRAF V600E Mutation PMA 2011 Vemurafenib Melanoma ALK Fusion PMA 2011 Crizotinib NSCLC EGFR Mutation LDT 2003 Gefitinib, Erlotinib NSCLC PMA 1998 PMA 2008 Trastuzumab Breast Cancer PMA 2005 Imatinib, Dasatinib, Nilotinib CML Source: Clin Chem 2013;59:198–201. cer cells. They can convert from being stem cell-like mesenchymal cells to epitheliallike cells and back and forth. The stem celllike cells have different responses than the epithelial-like cells,” he said. “The number of genes we’re picking up sometimes is in the hundreds for a single cancer. Most are important for growth and survival of the cancer, but only a few are driving the cancer. We don’t know how to identify those yet.” A Sketchy Track Record Cancer’s convoluted pedigree is reflected in the sketchy track record of the oncology therapeutics industry. Up to 70% of late-stage cancer trials ultimately fail to show benefit, and arrive at this unsatisfying conclusion after involving thousands of patients, costing as much as $1 billion, and taking 10 years of rigid adherence to strict clinical trial regulations and protocols. Yet recent breakthroughs twinning molecular diagnostics and therapies are spurring the industry to work smarter in weeding through candidate drugs and better matching new and existing therapies to patients more likely to benefit from them. Within just the past few years, mutation analysis as a precursor to targeted therapy has become the standard of care for certain tumor types. For example, the U. S. Food and Drug Administration (FDA) has approved trastuzumab for breast cancer patients with HER2 overexpression, and cetuximab and panitumumab for patients with KRAS-wild type metastatic colon cancer (See Table, above). Adding to the handful of tests used to guide clinical decision-making around chemotherapy, two developments in 2011 really captured the industry’s attention. In a first for the agency, FDA granted landmark approvals for two drugs and companion diagnostic testing required for patients to receive the drugs. First came approval for BRAF V600E mutation testing in patients with metastatic melanoma as a precursor to receiving vemurafenib, and then days later FDA approved ALK gene rearrangement testing in patients with late-stage non-small cell lung cancer (NSCLC) as a condition of receiving crizotinib. As significant as these approvals were, they illustrate just how far the industry has to go to achieve personalized treatment based on the patient’s tumor biology, and For Further Information ® Barrett JC, Frigault MM, Hollingsworth S, Miller GA, et al. Are companion diagnostics useful? Clin Chem 2013;59:198–201. Serum Proteins Alpha-1 Acid Glycoprotein Alpha-1 Anti-Trypsin Alpha-1 Microglobulin Haptoglobin IgA, IgG, IgM Transferrin Kidney Function Cystatin C ® Kelloff GJ, Sigman CC. Cancer biomarkers: Selecting the right drug for the right patient. Nature Reviews 2012;11:201–14. ® Modur V, Hailman E, Barrett JC. Evidence-based laboratory medicine in oncology drug development: From biomarkers to diagnostics. Clin Chem 2013;59:102–9. ® Parkinson DR, Johnson BE, Sledge GW. Making personalized cancer medicine a reality: Challenges and opportunities in the development of biomarkers and companion diagnostics. Clin Cancer Res 2012;18:619–24. ® Poste G, Carbone DP, Parkinson DR, et al. Leveling the playing field: Bringing development of biomarkers and molecular diagnostics up to the standards for drug development. Clin Cancer Res 2012;18:1515–23. ® Sölétormos G, Duffy MJ, Hayes DF, et al. Design of tumor biomarker- FAX: 206-575-8094 monitoring trials: A proposal by the European group on tumor markers. Clin Chem 2013;59:52–9. www.k-assay.com Clinical Laboratory News January 2013 LDT CML: Chronic Myeloid Leukemia; LDT: Laboratory Developed Test in CLIA certified clinical laboratory; NSCLC: Non-Small Cell Lung Cancer; PMA: Pre-Market Approval by the FDA 12779 Gateway Drive, Seattle, WA 98168 2 Indication BCR-ABL Translocation KAMIYA BIOMEDICAL COMPANY 2011.09 CLN Immunoassay.indd 1 Drug HER2 Amplification Diabetes / Stroke Alpha-1 Microglobulin Fructosamine Hemoglobin A1c Insulin Microalbumin 206-575-8068 Year CDx approval KRAS Mutation ** For Research Use Only. Not for use in diagnostic procedures in the U.S. 800-526-4925 Type Biomarker 8/5/2011 5:05:28 PM why new drug and biomarker development paradigms are needed, according to Parkinson. “We have applications of all these new technologies and glimpses of just how useful they can be in matching patients with their therapeutics. Yet we have many challenges, including developing new methodologies and standardizing them if they’re going to be released into clinical medicine.” In an opinion piece, Parkinson and his coauthors noted just how rare these parallel diagnostic and therapeutic regulatory approvals were, and in a nod to cancer’s complexity, they suggested that the value of selecting patients with either the BRAF V600E or ALK mutations is limited because “not all patients respond even within these marker-selected enriched patient groups, and the responses achieved vary significantly in extent and duration” (Clin Cancer Res 1012;18:619–24). As many as 60% of melanoma patients have the BRAF V600E mutation, but at most only about 7% of lung cancer patients have the ALK gene rearrangement. The latter suggests that conventional drug development strategies, based on measuring the effects of the drug in unselected patients with the type of cancer in question, probably would not have arrived at this biomarker-drug connection, because such a large number would have had to have been enrolled to detect a preferential response in this subset of patients. 2010, is testing 10 biomarkers and up to 12 different drugs from multiple companies, and is designed to enable investigators to incorporate knowledge gained during the trial into the trial while it is still ongoing. Patients’ estrogen receptor (ER), progesterone receptor, and HER2 receptor status, and their MammaPrint scores are being used to enroll and randomize them initially, and to stratify them within each arm of the study. When the patients have surgery, their tumor response is assessed and evaluated for biology-specific associations. At this point, the adaptive part of the trial comes into play. “Let’s say we find that type 1 breast cancer based on biomarker signatures responds particularly well to drug 2, and type 2 cancer biology responds well to drug 1. As the trial continues, patients with those particular biology types will be preferentially randomized into the trial arms where there’s a high likelihood of response while the control arm still receives all biological types. This is all based on predefined statistical significance,” explained Van’t Veer. “Our end point is pathological complete remission with a threshold of 85 percent predicted likelihood of success in a 300 patient Phase 2 trial. This means 100 to 200 patients are needed for each arm with a minimum of 60 to find successful drugbiomarker combinations or a failure.” Building Evidence for Biomarkers At the same time that I-SPY 2 is helping identify which investigational drugs lead to pathological complete response, the research team also expects the trial will advance evidence around biomarkers, potentially moving some from strictly experimental status to being on the pathway for FDA clearance (See Table, p. 4). For instance, Van’t Veer’s lab developed a gene expression signature that in experimental systems predicted response to a PARP inhibitor, and I-SPY 2 will test whether that experimental finding holds in patients. Efforts like this will be crucial going forward as more mutations are found with putative associations to various malignancies and therefore certain treatments, according to Vijay Modur MD, PhD, chief medical officer at HTG Molecular Diagnostics in Tucson. “It’s exceedingly important to generate clinical evidence so that we don’t pocket every mutation in the same space and say the same drug works with all. For example, we know that in melanoma, treatment of BRAF mutations with BRAFtargeted therapy results in a dramatic response. But for colorectal cancer, at least in preliminary studies, the response doesn’t See Cancer Diagnostics, continued on page 4 New Models for Clinical Trials Given the caveats of not only the BRAF V600E-vemurafenib and ALK-crizotinib stories but also other molecular diagnostic tests used in oncology clinical decisionmaking, researchers are pursuing new clinical trial designs. The goals of these efforts are many, including learning earlier in the drug development pipeline which agents will fail or succeed, speeding approvals for the drugs that succeed, predicting which patients will benefit from therapy, and personalizing the use of biomarkers and therapeutics. Without new and different paradigms, the oncology field will never speed up its knowledge turn, the time it takes for experiments to proceed from hypothesis to results and on to new hypotheses, according to Laura Van’t Veer, PhD, Angela and Shu Kai Chan endowed chair in cancer research at the University of California San Francisco. “There are more than 800 targeted investigational agents in development and if we keep doing the traditional way of testing drugs in Phase 1–4 trials where the Phase 3 trial would be a large randomized adjuvant trial, we will continue to need several thousands of patients to prove that a new drug is better than the standard. We’ll also continue to wait five-to-10 years for each drug to come to its endpoint. We need to screen drugs more quickly,” she explained. Van’t Veer is an investigator with I-SPY 2, one of the most notable in a new breed of clinical trials utilizing an adaptive design based on Bayesian statistics. This groundbreaking $26.5 million, 5-year study involving 20 major cancer centers and sponsored by the Biomarker Consortium, is testing whether adding novel agents to standard chemotherapy in the neoadjuvant setting improves outcomes in women with high-risk, fast-growing breast cancer. The trial, which was launched in Clinical Laboratory News January 2013 3 Adaptive Trials Not a Total Solution Clinical Laboratory News Cancer Diagnostics, continued from page 3 Editorial Staff Editor—Nancy Sasavage, PhD Senior Editor—Genna Rollins Senior Editor—Bill Malone Editorial Assistant—Christine DeLong Contributor—Chamindie Punyadeera, PhD Board of Editors Chair—Lorin M. Bachmann, PhD, DABCC VCU Health System, Richmond, Va. Members—Joshua Bornhorst, PhD University of Arkansas, Little Rock, Ark. Andrew Don-Wauchope, MD Juravinski Hospital and Cancer Center, Hamilton, Ontario Jacqueline Fisher, MS, C(ASCP) Abbott Diagnostics Division, Boston, Mass. Steven Goss, PhD Siemens Healthcare Diagnostics, Newark, Del. Pamela Steele, PhD Covance, Inc., Indianapolis, Ind. AACC Officers President—Robert H. Christenson, PhD, DABCC, FACB President-Elect—Steven H. Wong, PhD, DABCC, FACB Treasurer—Michael J. Bennett, PhD, DABCC, FACB Secretary—Elizabeth L. Frank, PhD, DABCC, FACB Past President—Greg Miller, PhD, DABCC, FACB Advertising SALES Scherago International, Inc. 525 Washington Blvd, Ste. 3310 Jersey City, NJ 07310 Phone: (201) 653-4777, Fax: (201) 653-5705 E-mail: aacc@scherago.com President—H.L. Burklund V.P. of Sales—Mike Minakowski Traffic Manager—Olga Guerra SUBSCRIPTIONs American Association for Clinical Chemistry, Inc. 1850 K Street, NW, Suite 625 Washington, DC 20006 Phone: (202) 857-0717 or (800) 892-1400 Fax: (202) 887-5093 E-mail: custserv@aacc.org Subscriptions to Clinical Laboratory News are free to qualified laboratory professionals in the United States. AACC members outside the U.S. pay $87 for postage. The subscription price for those who do not qualify for a free subscription is $87/year in the U.S. and $130/ year outside the U.S. For more information, contact the AACC Customer Service Department at (800) 892-1400 or (202) 857-0717 or custserv@aacc.org. editorial correspondence Nancy Sasavage, PhD, Editor Clinical Laboratory News 1850 K Street, NW, Suite 625 Washington, DC 20006 Phone: (202) 835-8725 or (800) 892-1400 Fax: (202) 835-8725 E-mail: nsasavage@aacc.org Contents copyright © 2013 by the American Association for Clinical Chemistry, Inc., except as noted. Printed in the U.S.A. seem to be the same, even if the mutation is the same. In an opinion article in Clinical Chemistry’s January 2013 special issue on cancer, Modur argued that in addition to a need to demonstrate clinical utility, both FDAcleared and lab-developed companion diagnostics have room for analytical improvement. Even FDA-cleared tests, like ER, HER2, and BRAF, have large inter-lab and patient variabilities. Modur and his co-authors called for improved and more flexible approaches to developing oncology companion diagnostics to respond to how quickly evidence is being generated around matching the right patients with the right therapy. The Downsides of Adaptive Trials Given the urgent need to press on with companion diagnostic development, the oncology field can’t—and isn’t—relying only on the adaptive trial model to make leapfrog advances toward personalized cancer therapy. That’s because I-SPY 2 and trials like it, including BATTLE in NSCLC, are logistically challenging and perhaps not as well-suited to less prevalent cancers, according to Meric-Bernstam. “This is a really outstanding model; however, it’s not doable on a small scale and it’s logistically difficult. If you want to give more than one drug you need to have multiple drugs available potentially from multiple different companies that may or may not be enthusiastic about comparison of their compounds to each other. One also needs a large enough pool of patients to draw from, as well as the commitment to prioritize this trial over all else to ensure that it is successfully recruiting so that all arms remain relevant while the trial is ongoing,” she explained. “Those are not easy things to accomplish, and that’s why only a handful of trials will be done like this.” I-SPY 2 also required special involvement from the FDA to consider this new paradigm, including using pathological complete response rather than long-term outcomes as the primary endpoint. The agency has acknowledged that diagnostic tests used to measure biomarkers can have a role in the selection of patients who are The Vanguard of Trial Enrollment To continue advancing molecular diagnostics and drug development for patient populations and cancer types not suited to adaptive clinical trial models, leading cancer centers, such as MD Anderson and Memorial Sloan-Kettering Cancer Center in New York City, have implemented protocols for performing molecular subtyping on at least certain populations, and using this information to enroll patients in other clinical trials. MD Anderson has two such initiatives, its clearinghouse and unusual responder protocols. Under the former, patients with advanced disease undergo molecular testing with a 46-gene panel looking for common cancer mutations. This information is used to assign patients to any relevant clinical trials the institution is involved in. If this process fails to uncover anything significant, the next step is targeted exome sequencing. “This is done in the research setting. The protocol is set up so that if an abnormality is identified and there’s a clinical trial that would be relevant, then the treating oncologist can order CLIA validation testing for clinical trial selection,” said Meric-Bernstam. Biomarker Categories in the I-SPY 2 Trial The landmark I-SPY 2 trial is testing 10 biomarkers and up to 12 different drugs. The trial’s novel adaptive design aims to enable investigators to incorporate knowledge gained during the trial into the trial while it is still ongoing. In addition to determining which therapies work best with particular cancer biology types, I-SPY 2 investigators also expect the trial to advance evidence around biomarkers, potentially moving some from strictly experimental status to being on the pathway for FDA clearance and widespread clinical use. Biomarker Category Qualifying biomarkers Exploratory biomarkers Hypothesis testing Hypothesis generating *FDA cleared or approved **Biomarker investigational device exemption (IDE) by the FDA as part of investigational new drug application facilitates companion diagnostic pre-market approval Source: adapted from and used with permission of Laura Van’t Veer, PhD @CLN_AACC 4 Purpose in I-SPY 2 Established biomarkers*Trial stratification, randomization IDE biomarkers** Clinical Laboratory News (ISSN 0161-9640) is the authoritative source for timely analysis of issues and trends affecting clinical laboratories, clinical laboratorians, and the practice of clinical laboratory science. likely to respond to specific therapies. It also has recommended using analytically validated biomarkers that have strong evidence of being fit for purpose to evaluate patient response to therapy, toxicity, and drug resistance. The agency’s commitment to revisit existing regulatory requirements given recent scientific advances is encouraging, but Parkinson contended that still more regulatory clarity and even new business models for the molecular diagnostics industry are needed. “The FDA, not inappropriately, has said these tests are going to drive the use of regulated therapeutics, therefore we intend to use our enforcement discretion and regulate them. We’re starting to see some guidance, but there still is confusion with regard to regulatory interfaces and the need for them or not. This is leading to a lot of uncertainty around business models and hesitancy about putting new financing into molecular diagnostic test companies.” MD Anderson’s unusual responder protocol is for patients such as those experiencing an unexpected rapid progression. Here, the focus is on deep characterization of patients’ tumors to identify mechanisms of resistance and predictors of response. Clearly these approaches are on the vanguard and not ready for widespread adoption. Indeed, sequencing performed in MD Anderson’s research labs for both its clearinghouse and unusual responder protocols are supported through philanthropy. However, clinical laboratorians across the country will do well to keep abreast of these efforts as this is the direction in which oncology is moving, according to Marc Ladanyi, MD, William J. Ruane endowed chair in molecular oncology and attending pathologist on the Molecular Diagnostics Service at Memorial Sloan-Kettering Cancer Center. “It’s a rapidly evolving field. Everybody assumes we’ll end up with these allpurpose assays that will sequence all the major cancer genes. Things are moving very quickly and it’s quite possible that within one-to-three years labs that haven’t gotten into tumor sequencing yet might be able to leapfrog some of these growing pains and go from multiple single-gene tests to more comprehensive testing platforms that are being developed,” he said. Modur sees reasons for all clinical labs, even those not currently performing tumor sequencing, to keep their dials tuned to this ever-changing scene. “Laboratorians should be able to have discussions with oncologists to determine what the laboratory test menu is going to look like, both for established and qualifying markers. Many oncologists already are requesting that their patients be tested for qualifying biomarkers because the level of evidence for some of them is pretty high,” he explained. “Then there are exploratory biomarkers that are driving clinical trials and may be performed without clinical laboratory oversight right now. The field is moving away from that because many trial protocols pre-specify that even exploratory biomarkers have to be done in a CLIA regulatory environment. So laboratorians need to understand the spectrum of biomarkers, categorize them, and have an educated discussion with oncologists on where each one falls and how the clinician’s CLN demands for testing can be met.” Clinical Laboratory News January 2013 Payment Uncertainty Could Last a Year Molecular Test Coding, from page 1 even more uncertainty over the fate of molecular testing. According to lab coding expert Charles Root, PhD, Medicare contractors have their work cut out for them as they come up with payment for the new codes, as most have little expertise in molecular diagnostics. “Ultimately, the only way labs will really know what they will be paid in January will be to submit a claim to Medicare and see what comes back,” he said. The founder and president of CodeMap, LLC, Root spoke as part of AACC’s Reimbursement and Regulatory Update webinar on November 27. Flying in the Dark Overall, the shift to the new coding system looks like an improvement to labs and diagnostic companies, but the devil is in the details, emphasized industry consultant Rina Wolf. “This should be good news. But the whole pricing issue is absolutely key, because we’re totally flying in the dark right now,” she said. “We don’t know what this is going to mean from any of the payers, whether from CMS or the commercial payers, who usually follow the lead of CMS.” Wolf is vice president of commercialization strategies, consulting, and industry affairs at XIFIN, a revenue cycle management solutions company. At CLN press time in mid December, CMS had yet to offer any instructions to its contractors on how they should go about paying claims with such a short window to gap-fill their pricing. Wolf urged CMS to take a balanced approach that would allow contractors to make informed decisions. “I think it’s very important for CMS to balance our need to get the answers on pricing with the ability to get the job done in a considered and appropriate way,” she said. “This is one of the biggest issues facing the business of clinical laboratories right now, and the business of the lab directly affects patient care. Some diagnostic companies run on such incredibly slim margins that this will have a huge impact on their ability to survive.” Some contractors have almost nothing to go on for pricing the new codes, due to the way the previous system of stacking multiple codes for each test obscured what was being paid for. “With the old way of using code stacks, some of these contractors probably weren’t even aware that they were seeing claims for certain tests,” Wolf said (See Box, right). Even the contractor with unquestionably the most experience in molecular diagnostics, Palmetto GBA, would have a lot of work to do before rolling out a new pricing scheme, explained Elaine Jeter, MD, the medical director of Palmetto’s Molecular Diagnostics Services Program (MolDX). “The time is short and a lot of information is required,” she said. “We don’t have any information on the resources required for an assay, only what the labs have been billing.” Palmetto’s MolDX program covers Medicare claims in California, Nevada, and Hawaii. One reason that experts worry about CMS’s choice of gap-filling is that CMS has employed this method infrequently and imperfectly. In most cases, CMS prices new codes by borrowing prices from existing, similar codes in a centralized fashion. This simpler, kinder method is called crosswalking. “There are usually 10 to 20 new lab codes every year, and CMS has in the past only used gap-fill for a test about once every 4 years, so it’s been very exceptional for any test to go through the gap-fill process,” said Bruce Quinn, MD, PhD, senior health policy specialist at Foley Hoag, LLP. “The only one in my nine years of experience with Medicare was for HbA1c measured directly in the office, and that was very messy. A lot of contractors were uncertain about how to price it, and local prices varied almost by a factor of three. Finally, Congress stepped in and decided what the payment would be, effectively canceling the gap-fill process.” Private payers are in a jam, too. They depend on Medicare’s published price list, which no one expects for some time. And even when CMS does give contractors parameters to begin gap-filling, commercial payers will still have to sort out which contractor they will follow if prices vary across the country, Wolf noted. “This is of tremendous concern to diagnostic companies. Of course, the industry wants to provide the best patient care, but these are also busi- 2013 Molecular Reimbursement Timeline This year, hundreds of new CPT codes created for molecular diagnostics must replace the old code stacks labs used for more than a decade. The government surprised labs and diagnostic companies when it announced late last year that it would not use the same prices as the old codes, but rather ask its local contractors to build their own pricing schemes through a little-used process called gap-filling. Under final Medicare regulations announced in November, final prices for molecular diagnostics will remain in flux until January 2014. Between now and then, CMS will let each contractor set its own prices and take an average of these as the maximum allowed price, or National Limitation Amount, going forward. ®By April 30, CMS posts interim contractor-specific amounts online ®A 60-day comment period on interim amounts ® CMS posts final contractor-specific amounts and National Limitation Amounts (NLA) online ® CMS sets the NLA for each CPT code at the median of the contractorspecific amounts ®Reconsideration requests accepted for 30 days ®Final NLAs become effective January 1, 2014 for the entire country Source: Charles Root, PhD, CodeMap, LLC 6 Clinical Laboratory News January 2013 New Molecular Codes Take Effect for 2013 Despite uncertainties about how they will be reimbursed, the new molecular codes that come into full effect this year solve some long-standing problems. Without unique, analyte-specific codes for molecular tests, labs have for decades cobbled together their own lists of other codes that describe the various steps of performing the test. For labs, this led to headaches with denied claims or underpayment. For payers, it meant they never really knew which tests they were paying for or what the tests really cost, since all they could track were the fragments of each lab’s unique bundle of codes, rather than the whole. Perhaps just as significant, claims data have been almost worthless for tracking physicians’ utilization of these advanced tests. A white paper from UnitedHealthcare’s Center for Health Reform and Modernization noted as an example that under the old coding system, the genetic test for Canavan disease required six steps and thus six procedure codes. However, labs also used these same procedure codes for genetic tests for five other diseases, including cystic fibrosis and Tay-Sachs, leaving the payer unable to distinguish one test from the other. In 2011, the American Medical Association (AMA) released its overhauled coding system for molecular tests with a new tiered approach that featured analyte-specific codes. The Centers for Medicare and Medicaid Services (CMS) essentially outsources this work to AMA, but in this case could not decide how to implement AMA’s work. AMA, as well as the College of American Pathologists (CAP), pushed CMS to make molecular coding and reimbursement a pathologist-centered rather than a lab-centered system, arguing that molecular tests require a pathologist’s interpretation and should reside on the physician fee schedule. Diagnostic companies and many others in the lab community welcomed the news late in 2012 that CMS would keep the new codes on the lab fee schedule for 2013, according to Rina Wolf, vice president of commercialization strategies, consulting, and industry affairs at XIFIN, a revenue cycle management solutions company. “We believe that this is good news because our contention has been that these are not new tests, these are new codes for existing tests for which the components all previously were on the clinical lab fee schedule,” she said. “We also believe that the majority of these tests do not require additional pathology interpretation. CMS did acknowledge that, and provided a temporary code, G0452, for those circumstances where an ordering physician believes that it is appropriate to have an additional pathology interpretation.” Under the new coding system, tests that make up the majority of the volume of molecular diagnostics fall into the first tier of a two-tier system. Tier 1 is home to some 100 codes that together represent more than 90% of the volume of molecular tests currently performed. Tier 2 is reserved for low-volume, esoteric tests, within which are nine buckets based on test complexity. A different CPT code is reserved for each of the nine levels. Within each level, many different tests can reside. Notably, it is up to AMA to list which particular tests can be coded under each level—labs cannot selfassign. Labs have to be sure that AMA has listed the particular test they use under one of these codes in order to bill Medicare. nesses that have to survive so that they can do that. With no clarity on pricing, they can do no revenue modeling for 2013—they don’t even know if they’ll be able to afford to do these tests anymore.” According to Root, gap-filling allows contractors to use four methods to calculate pricing: what is charged for each test; the resources and actual costs of performing the test; payment amounts determined by other payers; and the payment amounts or resources required for comparable tests. CMS may give contractors some direction on which of these methods they should use. However, because the previous coding system concealed what contractors were paying for, it seems likely the first two options would be very difficult. “My own opinion is that most contractors will be looking to see what Palmetto does,” Root said. “They will be the leader here and should have the most data on what they’re actually paying for right now.” In either case, CMS will not even publicly post interim contract-specific amounts until April (See Box, left). Palmetto Takes Center Stage As Medicare’s contractors try to make sense of how to price the new molecular codes, all eyes will be on Palmetto GBA, the first and only contractor to develop its own program specifically to address the burgeoning field of molecular diagnostics. Palmetto’s MolDX program requires each test to carry a unique identifier. This way, Palmetto can keep track of which tests it pays for and how they are used. With the new ability to recognize these tests for what they are, MolDX also requires a technology assessment before deciding on whether it should be covered under Medicare as “reasonable and necessary.” The technology assessment looks at analytical and clinical validity as well as clinical utility. Since Palmetto is the only entity in Medicare that appears to have a grasp of molecular testing, some experts believe that the best route for CMS would be to adopt MolDX as a national program. According to Mike Barlow, vice president of Palmetto’s Jurisdiction 1 operations under which MolDX operates, such a move would be easier said than done. “We’ve had discussions with CMS about this since we have a very successful program with demonstrated results,” Barlow said. “At the same time, CMS has limited options on this within the regulations.” Not to be confused with the new CPT codes, MolDX unique identifiers go a step further in that they differentiate between different companies’ FDA-approved tests or different labs’ laboratory-developed tests (LDTs). The new CPT codes, developed by the American Medical Association (AMA), are analyte-specific, so they’re blind to methodology—and to a lab or company’s particular assay. Although the old code stacking system created problems for both payers and labs, molecular tests under the new codes will still require unique identifiers and technology assessments under MolDX, Jeter noted. “Code stacking was worse, but there are still gaps,” she said. “Without the unique identifiers, we still don’t know what we’re paying for.” For example, Jeter noted that for BRAF gene testing, three FDA-cleared tests exist, and at least 25 LDTs. Under the old system, each FDA-cleared test and most of the LDTs billed Medicare with unique code stacks. Although the new molecular CPT codes will fix that problem, they still don’t differentiate among the 30 or more assays for BRAF, as they’d all share the same analyte-specific code. On the other hand, just because AMA has assigned a new CPT code to a test does not mean MolDX will automatically cover it, Jeter emphasized. Certain esoteric, lowvolume tests will reside on a second tier of CPT codes under the AMA system that are not analyte-specific and coded by level of complexity, offering even less transparency. “ApoE is an assay in Tier 2, Level 2. Since this assay has no proven clinical utility, how will other payers separate this non-covered assay from others in Tier 2, Level 2 that warrant coverage?” Jeter said. “With unique identifiers, Palmetto GBA is able to create edits to deny coverage for ApoE, yet appropriately pay for other assays in Tier 2, Level 2.” Even if CMS chooses not to adopt Palmetto’s MolDX program nationally for Medicare, private payers are eager to capitalize on MolDX’s work. According to Wolf, several payers have approached Palmetto requesting access to their database of unique test identifier codes. “It’s the opinion of many of us in the diagnostics industry that this could be a good thing,” Wolf said. Multi-analyte Algorithmic Tests in Limbo In rolling out the new molecular codes, AMA also created codes for a new breed of tests that use multiple analytes and an algorithm to produce a result, such as a risk score. In some cases, the algorithms are proprietary. Laboratorians might be familiar with the Food and Drug Administration (FDA) term for these tests, In Vitro Diagnostic Multivariate Index Assays (IVDMIA). AMA’s term, adopted by CMS, is Multi-analyte Assays with Algorithmic Analysis (MAAA). For now, CMS will not pay for these tests and their codes will not appear on Medicare fee schedules. According to CMS, “Medicare does not recognize a calculated or algorithmically derived rate or result as a clinical laboratory test since the calculated or algorithmically derived rate or result alone does not indicate the presence or absence of a substance or organism in the body.” CMS prefers to pay only for the individual component tests that feed into the algorithm, but not the MAAA as a whole. According to Root, when CMS doesn’t recognize codes, commercial payers usually will not either, making reimbursement that captures the full value of MAAAs highly uncertain. “CMS is sustaining its policy that algorithms are simply arithmetic, no matter how sophisticated, and have no value in reimbursement,” he said. “Obviously, this is contrary to fact in some cases, because some algorithms have cost millions of dollars to develop and may be extremely expensive. And they will probably be licensed as well—that’s the only way the developers will get any money back—so a laboratory that licenses the use of a multianalyte panel will not get Medicare payment for that use of the algorithm.” More Pain for Pathology Labs If pathologists saw CMS’s decision to put the new molecular codes on the clinical lab fee schedule as a loss, they should prepare for even greater worries ahead when it comes to reimbursement for traditional anatomic pathology services. Depending on how often a pathology lab performs cer- tain procedures, a cut this year to a single code—88305—could have a big impact. Pathology labs frequently use this code to bill for preparing slides, representing about $1.5 billion a year for Medicare. The 2013 physician fee schedule slashes payment for the lab work involved in this code by 51%. As with many pathology codes, the code is used for both the pathologist’s interpretation as well as the labor of other lab professionals and supplies needed to prepare the slide. For 2013, the lab portion of this payment is $33.70, down from $69.78 in 2012, according to Root. Even though CMS increased the payment slightly for the professional interpretation element of this code, it could have a big impact on certain labs, depending on what constitutes the core of their business, explained Quinn. “The affect of this cut will be somewhat selective. If you were running a shop that performed thousands accuracy of calibrators depends on accuracy of spiking solutions DID YOU KNOW Quality and characterization of critical starting materials, difficulty in material handling, and weighing technique can affect calibrator accuracy. Our shelf stable Snap-N-Spike® format eliminates painstaking weighing operations – providing the ability to spike a known concentration of analyte into your matrix of choice. Cerilliant… when results matter of skin biopsies and prostate biopsies, then it would be a huge impact. But if that code only represented two percent of your business, it might not matter much.” A Waiting Game According to experts, labs are limited in how they can plan for reimbursement changes coming with the new codes. Labs must use the new CPT codes and make sure the old codes are deactivated in their billing systems, Root noted. As CMS and local Medicare contractors negotiate a way through the gap-fill process, labs should keep in close contact with their own contractor and make sure they receive e-mail CLN and other updates. For more about how budget negotiations in Congress will affect labs, see a special article on page 11 by AACC government affairs director, Vince Stein, PhD. For a complete list of our Certified Spiking Solutions® for Clinical Applications, visit Cerilliant.com • Analgesics • Antidepressants • Antiepileptics • Antipsychotics • Caffeine-related Drugs • Cardiac Drugs • Catecholamines • Hormones — — — — — Female Male Neonatal OH Vitamin D Thyroid • Immunosuppressants • NSAIDs • Vitamins — Heather Lima, PhD SYNTHESIS OPERATIONS Visit Cerilliant.com or call: 800/848-7837 USA or CANADA 512/238-9974 INTERNATIONAL Cerilliant Quality ISO GUIDE 34 ISO/IEC 17025 ISO 13485 ISO 9001 GMP/GLP © 2013 Cerilliant Corporation CLN 1/13 Clinical Laboratory News January 2013 7 CLN’s improving healthcare through laboratory medicine series Saliva An Often Forgotten, but Convenient Diagnostic Fluid By Chamindie Punyadeera, PhD F or decades, dental healthcare professionals have measured the buffering capacity and bacterial content of saliva to assess a person’s risk of developing tooth decay (1). Today, scientific and technological advances in biochemistry, microbiology, and immunology are leading to the discovery of new biomarkers in saliva that can be used to detect systemic illnesses such as ischemic heart disease and heart failure (2–4) and cancer (5).This growing recognition of the association between an individual’s oral and overall health has led to renewed interest in using saliva as a diagnostic fluid. In fact, human saliva offers several benefits compared to traditional blood-based biochemical analyses. Saliva collection is non-invasive and therefore stress-free for patients. For the person collecting the sample, saliva also poses minimal risk for contracting infectious diseases such as HPV, HCV, and HIV. Finally, saliva is an ideal biofluid for developing countries due to the low cost of collecting and processing samples (Figure 1). This article describes the current state of knowledge for saliva diagnostics and the obstacles that researchers, manufacturers, and laboratorians will need to overcome before saliva is widely accepted as a reliable diagnostic fluid. Three major glands—the parotid, submandibular, and sublingual—and about 400 minor glands located within the oral cavity produce saliva. A healthy adult produces an average of 500–1,500 mL saliva/ day at a rate of approximately 0.5 mL/ min. Human saliva has a multitude of functions within the oral cavity, including: maintaining homeostasis; promoting Human saliva harbors a repertoire of proteins, lipids, RNA, DNA, and some 700 microbial species. To move toward less invasive clinical management of patients, researchers have been pursuing saliva as a biofluid for early disease detection and prognosis, risk stratification, and monitoring treatment response. In fact, saliva has already been used as a biological fluid for diagnosis and prognosis of oral, head, and neck cancers, periodontal diseases, diabetes, and autoimmune disorders. Researchers also have found other diagnostic uses for saliva. One research team identified biomarkers in saliva for detecting early-stage pancreatic cancer (9), and another measured soluble c-erbB-2Her2/neu wound healing; lubricating the oral cavity; facilitating mineralization of dental surfaces; digesting carbohydrates by salivary α-amylase; digesting lipids via salivary lipase; and facilitating chewing, speaking, swallowing, and taste perception (6, 7). While the human mouth provides good growth conditions for many microorganisms, the anti-microbial properties of saliva also help maintain oral hygiene by clearing and inhibiting growth of microorganisms (8). levels in saliva collected from breast cancer patients and concluded that c-erbB may be useful in detecting and monitoring recurrence of this disease (10). Current research also is producing valuable datasets for further analysis. Salivaomics is an open-access database (www. hspp.ucla.edu) that contains salivaomicsbased studies and includes information on the biology, diagnostic potential, pharmacogenomics, and pharmacoproteomics of saliva. A Saliva Primer 8 Clinical Laboratory News January 2013 Saliva Proteome Human saliva is a plasma ultra filtrate and contains proteins either synthesized in situ in the salivary glands or derived from blood. It contains biomarkers derived from serum, gingival crevicular fluid, and mucosal transudate. To date, researchers have identified 2,340 proteins in the salivary proteome, of which 20–30% are also found in blood (11), an encouraging indicator for the clinical utility of saliva as a diagnostic fluid. In contrast to the plasma proteome, in which 99% of the total protein content is contributed by 22 highly abundant proteins (8), the 20 most abundant proteins in human whole saliva (WS) constitute only 40% of the protein content (12). This composition suggests that detecting biomolecules of clinical sensitivity and specificity in saliva should be feasible and easier than in blood. Unlike the plasma proteome, however, the WS proteome is highly susceptible to a variety of physiological and biochemical processes, such as salivary protein modifications occurring in the mouth that are catalyzed by host and bacterial derived enzymes. Such modifications also could present unique challenges for clinical saliva proteomics. The dynamic range of proteins in saliva is another challenge. For instance, a-amylase, an abundant protein in saliva, is present at mg/mL concentrations, while the IL-6 and IL-8 cytokines of potential clinical relevance are present at concentrations of only pg/mL. This disparity highlights the importance of developing tools and strategies for discerning low abundance proteins with clinical relevance in saliva. How molecules are transported from blood into saliva may also be important for successful use of saliva as a diagnostic fluid. Lipophilic molecules such as steroid hormones passively diffuse into saliva, while water and electrolytes pass through the pores of acinar cells. Various peptides in blood move through protein channels, and large proteins are transported via pinocytosis (4). Commercially Available Saliva Tests Two U.S. companies were early pioneers of oral diagnostics: Epitope, Inc. and Saliva Diagnostic Systems, Inc. They both commercialized saliva collection devices in the early 1990s, and in 1996 the Food and Drug Administration (FDA) approved Epitope’s Table 1 Figure 1 The Advantages and Disadvantages of Saliva as a Diagnostic Fluid Efficacy of Saliva Collection Methods Collection Device Drool cup Median/IQR Salimetrics oral swab Median/IQR Salivette cotton swab Median/IQR Salivette synthetic swab Median/IQR CRP (pg/mL) 28/9–51 9/5–30* 19/7–46 14/5–44 IgE (pg/mL) 72/38–202 72/35–123 43/30–153 37/30–164* Myoglobin (pg/mL) 98/31–140 23/2–54* 23/12–52** 59/34–136 Analyte The table summarizes data for the median and interquartile range (IQR) levels for three analytes collected by four different methods. We observed significant differences (*p<0.05) for both CRP and myoglobin levels using the drool cup versus the Salimetrics collection device, as well as significant differences (**p<0.01) for myoglobin using the drool cup versus the Salivette cotton swab. For IgE, the levels collected in the drool cup versus the Salivette synthetic devices were correlated more closely (*p<0.05) (14). Orasure HIV test, the first test that used oral fluid to test for an infectious disease. More recently, FDA approved the first over-the-counter salivary HIV test that allows people to test themselves in the privacy of their homes for the HIV virus. The OraQuick HIV test, which takes only 15 minutes from start to finish, detects the presence of HIV antibodies in saliva via mouth swab. Several companies outside the U.S. have commercial tests to detect drugs-of-abuse in a spit sample, including Cozart Biosciences, Securetec, and Mavand. Some of these companies send their kits via regular mail to customers, allowing individuals to collect their own saliva either in a cup or with a swab and then send the sample to a laboratory for analysis. Other tests target DNA in saliva. Canadabased DNA Genotek was the first company to commercialize a broad range of saliva collection tools for genotyping based on the polymerase chain reaction (PCR), microarrays, and sequencing. Oral DNA Labs, a subsidiary of Quest Diagnostics, also offers two salivary tests in the U.S. in its CLIA-approved testing facility. My PerioPath is a DNA test that determines the risk of periodontal infections by detecting bacterial pathogens in saliva. OraRisk HPV is a salivary test that determines an individual’s risk of developing HPV-related oral Figure 2 Saliva Collection Devices cancers. It identifies various HPV genotypes, including HPV 8, 11, 16, and 18. Emerging Clinical Applications Other applications of salivary diagnostics are emerging, including for the detection of cardiovascular disease (2–4) and head and neck cancer (5). Our group and others have demonstrated that salivary C-reactive protein (CRP) levels can be used as a biomarker to differentiate patients with ischemic heart disease from healthy controls (2, 3) (Figure 3). Work by Denver et al. also has demonstrated that salivary endothelin concentrations (13) and salivary natriuretic peptide levels (4) are useful as biomarkers to assess heart failure. In addition, we tested commercially available saliva collection devices (Figure 2) to determine their efficacy for detecting salivary proteins with varying molecular weights, such as CRP, myoglobin, and IgE, in healthy controls (14) (Table 1). Our data demonstrated significant differences in analyte levels based on the collection device. Early detection of head and neck squamous cell carcinoma (HNSCC), cancers that occur in the nasal passages, sinuses, mouth, larynx, and pharynx, is another focus of salivary diagnostics. Tobacco use is a major risk factor for this type of cancer, and 30–50% of HNSCCs are a direct result of HPV 16, and to a lesser extent HPV 18, infections. DNA methylation in cells is an early event that occurs during tumor initiation, and we have found methylation of three tumor suppressor genes (DAPK1, RASSF1A and p16) in saliva collected from HNSCC patients that is not present in healthy controls (5). Roadblocks to Advancement Shown here is the wide variety of commercially available saliva collection devices in use today: drool collected in a sterile specimen container (A); Salimetrics oral swab (B); Salivette cotton and synthetic device (C); Greiner Bio-One saliva collection system (D); OriGene DNA collection device (E); and DNASal collection device (F). Expansion of salivary diagnostics is hampered by several factors. First, analytes in saliva are usually present at only 0.1–0.001 of the levels found in blood; therefore, very sensitive detection technology is required to develop effective tests. Another impediment is the lack of information about baseline levels or reference ranges of molecules in saliva within a healthy control population. This information is crucial to discriminating disease-specific changes. To be clinically useful, there also must be reliable correlations between levels of the target substance in saliva and in blood or plasma. For example, we know that salivary Clinical Laboratory News January 2013 9 diagnostics are not well suited to measuring glucose levels because blood and salivary levels of this analyte are poorly correlated. This could be the case for other analytes as well. Another complication is that contamination of saliva samples with even a small amount of blood will lead to a false-positive result. In fact, bleeding after brushing or flossing occurs quite frequently and could contribute to high false-positive rates for salivary tests. Research also is needed on how levels of molecules vary diurnally. For example, we know that salivary growth hormone levels are higher in the morning than during the day, which could also be the case for other biomarkers. The lack of standardized saliva collection methods also makes the widespread adoption of salivary diagnostics more challenging. In one study, researchers reported that the OraSure saliva collection device detects hepatitis C virus with greater sensitivity than the Salivette device (15). Such differences may be related to the collection device itself. What Does the Future Hold? As our knowledge of the biomolecules present in saliva grows, the potential applications for oral and systemic disease diagnosis will expand. While the scientific link between salivary biomarkers and oral diseases is clear, more studies are needed to delineate the mechanisms by which saliva reflects other systemic diseases. Furthermore, before saliva Figure 3 Salivary CRP Levels A B A. Salivary CRP levels in healthy volunteers (n = 55) and in patients with ischemic heart disease (n = 28). B. Correlation of salivary to plasma CRP levels. can become widely recognized as a reliable diagnostic fluid, we need to more fully understand a number of important variables. First, we need to define the normal biological variability of biomolecules in saliva, such as diurnal rhythms, inter- and intra-subject variation, and age and gender effects. The influence of diet, medication, smoking, alcohol, and physical activity status may also influence levels of biomolecules in saliva. From an analytical stand- Simple and direct saliva collection with Evergreen’s new Oro-Col point, methodological variations caused by saliva sampling, handling, and storage conditions will need to be defined, as well as methodological variations due to the analytical techniques used. Since the salivary proteome is sensitive to both extrinsic and intrinsic factors, analyte reference ranges in saliva will need to be carefully documented. Salivary diagnostics has enormous potential for the future, but we need to lay a solid scientific foundation in the present in order to realize that potential. Non-invasive tests for detecting breast cancer, viral, and bacterial diseases, cardiovascular and metabolic diseases, and general nutritional deficiencies could make a tremendous impact on global health. The key parties responsible for translating salivary research from a laboratory setting to clinical practice, including scientists, regulatory agencies, and third parties such as insurance companies, will need to work together to determine how new saliva diagnostics are adopted by the health care community. But undoubtedly, a saliva swab test in the privacy of one’s home or at the general practitioner’s office will become a reality for diagnosing CLN systemic diseases. References • Simple,non-invasivesamplecollection. • Directcollectionprocessprovidinganoptimalamountof oralfluidsampleintoacalibratedvial. • Biomarkerconcentrationsinoralfluidsreflectthe concentrationsinblood. • Oralfluidhasbeenusedasthealternatesampleforthe detectionandmeasurementofdrugsofabuseandbiomarkersforvariouscancers,cardiacdiseases,stressand otherclinicalconditions. https://www.evergreensci.com info@evergreensci.com Web E-mail 323-583-1331 Tel Scan me for more information 800-421-6261 Toll-freetel 323-581-2503 Fax 2254 E 49th Street, Los Angeles, CA 90058 Mail 10 Clinical Laboratory News January 2013 1. Lawrence HP. Salivary markers of systemic disease: noninvasive diagnosis of disease and monitoring of general health. J Can Dent Assoc 2002;68:170–4. 2. Christodoulides N, Floriano PN, Miller CS, et al. Lab-on-a-chip methods for pointof-care measurements of salivary biomarkers of periodontitis. Ann N Y Acad Sci 2007;1098:411–28. 3. Punyadeera C, Dimeski G, Kostner K, et al. One-step homogeneous C-reactive protein assay for saliva. J Immunol Methods 2011;373:19–25. 4. Yang Foo JY, Wan Y, Kostner K, et al. NT-ProBNP levels in saliva and its clinical relevance to heart failure. [Epub] PLoS One October 31, 2012 as doi:10.1371/journal. pone.0048452. 5. Ovchinnikov DA, Cooper MA, Pandit P, et al. Tumour-suppressor gene promoter hypermethylation in saliva of head and neck cancer patients. Transl Oncol 2012;5:321–6. 6. Pfaffe T, Cooper-White J, Beyerlein P, et al. Diagnostic potential of saliva: Current state and future applications. Clin Chem 2011;57:675–87. 7. Mohamed R, Campbell JL, CooperWhite J, et al. The impact of saliva collection and processing methods on CRP, IgE, and Myoglobin immunoassays. CTM 2012;1:19. 8. Schulz BL, Cooper-White J, Punyadeera CK. Saliva proteome research: Current status and future outlook. [Epub ahead of print] Crit Rev Biotechnol May 21, 2012. 9. UCLA Newsroom. Researchers find biomarkers in saliva for detection of early-stage pancreatic cancer. http://newsroom.ucla.edu /portal/ucla/early-detection-biomarkersof-153212.aspx (Accessed August 2012). 10.Streckfus CF, Mayorga-Wark O, Arreola D, et al. Breast cancer related proteins are present in saliva and are modulated secondary to ductal carcinoma in situ of the breast. Cancer Invest 2008;26:159–67. 11.Bandhakavi S, Stone MD, Onsongo G, et al. A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J Proteome Res 2009;8:5590–600. 12.Loo JA, Yan W, Ramachandran P, et al. Comparative human salivary and plasma proteomes. J Dent Res 2010;89:1016–23. 13.Denver R, Tzanidis A, Martin P, et al. Salivary endothelin concentrations in the assessment of chronic heart failure. Lancet 2000;355:468–9. 14.Topkas E, Keith P, Dimeski G, et al. Evaluation of saliva collection devices for the analysis of proteins. Clin Chim Acta 2012;413:1066–70. 15.Judd A, Parry J, Hickman M, et al. Evaluation of a modified commercial assay in detecting antibody to hepatitis C virus in oral fluids and dried blood spots. J Med Virol 2003;71:49–55. Chamindie Punyadeera, PhD, is the lead research scientist in the Saliva Translational Research Group, Tissue Engineering and Microfluidics Laboratory, Australian Institute for Bioengineering and Nanotechnology at the University of Queensland in Queensland, Australia. Email: c.punyadeera@uq.edu.au Disclosure: The author has nothing to disclose. A Look Into the Crystal Ball—Budget Cuts, Healthcare Reform What Laboratory Professionals Can Expect in 2013 By Vince Stine, PhD T he 2012 presidential election is history, and the White House and Congress are back at work in Washington, D.C. By winning, President Barack Obama became the third incumbent President in a row to be re-elected and the fifth Chief Executive to do so since 1972. Similarly, voters returned a Democratic Senate and Republican House of Representatives, allowing Senator Harry Reid (D-Nev.) and Representative John Boehner (R-Ohio) to keep their respective leadership positions. Now as the new year begins, the two sides need to put aside the bitterness of the past year and work together to resolve a number of pressing issues facing the nation, particularly budgetary matters, that are likely to impact healthcare spending and clinical laboratories. Falling into the Abyss? At CLN press time in December, all of Washington was talking about the pending ‘fiscal cliff.’ This term, first coined by Federal Reserve Chairman Ben Bernanke, reflects his concerns about the impact of $700 billion in spending cuts and tax increases scheduled to take effect on January 1, 2013. The immensity of these budgetary changes could, according to the Congressional Budget Office (CBO), result in the “economy toppling back into recession.” This concern about the state of the economy has lawmakers scrambling to work out deals to develop a long-term deficit reduction package and enact legislation to retain most of the Bush-era tax cuts. The current crisis is a byproduct of the Budget Control Act of 2011 that Congress passed to forestall an earlier budgetary shortfall. This legislation required $1.2 trillion in across-the-board spending cuts over 10 years starting in 2013 and split evenly between domestic and defense spending. Although certain programs such as Medicaid, Social Security, and Veterans benefits were exempted from these reductions, Medicare was not. The law did allow Congress to replace the indiscriminate cuts with more targeted reductions prior to the effective date. Until now, Congress has been unable to do so. Another element of the fiscal cliff is the Bush-era tax cuts that are set to expire at the end of 2012. President Obama has proposed extending these tax benefits to families making $250,000 annually or less. According to the Citizens for Tax Justice, this limited extension would reduce federal revenues by $243 billion in 2013 and more in the following years. Congress is likely to pass some variation of the President’s offer, and this means the legislative branch will need to find additional revenues to pay for extending the tax cuts. Payment Reductions for Labs Looming Also looming as part of this fiscal nightmare is a projected 26.5% cut in Medicare physician payments. Past congressional efforts to restrict the growth in physician reimbursement created what many consider a formula that specifies annual deep reductions in physician payments. Now, every year Congress has to intervene to prevent these cuts. Legislators passed a $17 billion fix in 2012 that included a number of cuts to healthcare providers, including a 2% cut in the clinical laboratory fee schedule beginning in 2012. Another temporary fix in 2013 is expected to cost $25 billion, so legislators will have to find money to pay for this budgetary issue as well. Physicians aren’t the only ones facing pay cuts. Payment reductions in 2013 are coming for labs too as a result of the 2010 healthcare reform law. The reform package included two annual cuts for clinical laboratories: a permanent reduction in the clinical laboratory consumer price index (CPI) update by a “productivity adjustment” and a 5-year annual cut in the CPI update of 1.75% that expires in 2015. These cuts, combined with physician fix reductions, will cut laboratory payments by 2.95% in 2013. But this figure could increase to 4.95% if Congress fails to address the fiscal cliff and the across-theboard Medicare cuts take effect on January 1st as a down payment for deficit reduction. More on the Table Given the scope of the U.S. budgetary issues, Congress has even been discussing such sacred cows as reducing the mortgage interest and charitable tax deductions. In other words, everything is on the table, and it’s likely that clinical laboratories will be a target to contribute more. Lawmakers have a laundry list of potential items to choose from that will hurt laboratory budgets (See Box). In the short-term, Congress may agree to a 6-month delay to work out a longterm deal with the White House given the sensitivity and politics surrounding the issues. However, the window of opportunity is small because legislators will soon start thinking about the 2014 mid-term elections. Healthcare Reform Resurfaces Another issue that will receive a lot of attention in 2013 will be healthcare reform. Potential Congressional Targets for Cost Savings in Labs ®Laboratory co-payments for Medicare recipients. ®Competitive bidding for laboratory services. ®Extending the 1.75% annual cut in the lab consumer price index due to expire in 2015. ®Further reductions in the laboratory fee schedule. ®Bundling payments. Legislators are looking for ways to save money to pay for potential tax cuts and healthcare reform in 2013. Laboratory professionals should pay close attention to the issues and be proactive with their legislators. AACC’s Government Affairs Advocacy Network allows you to contact your elected officials and regulators to express your opinion on a variety of legislative and regulatory issues important to clinical laboratorians. Go to our website at www.aacc. org and visit the Government Affairs pages for more information. With the election over and Republicans conceding they are unable to repeal the law, policymakers will focus their attention on implementation of the act. Some of the more popular and easier to implement provisions of the law are already in effect, such as allowing children up to age 26 to remain on their parents’ insurance, barring the denial of insurance coverage to individuals under 19 due to pre-existing conditions, and expanding prevention and wellness benefits. Over the next 2 years, however, some of the more complicated, costly, and controversial provisions will go into effect. One difficult provision involves implementing state insurance exchanges. These local entities will be responsible for reviewing and approving qualified health plans, educating consumers and small businesses, and enrolling participants in the plans. To date, 17 states and the District of Columbia have agreed to create and manage such exchanges; however, nearly 20 states have declined to participate and the rest are undecided or partnering with the federal government. If a state chooses not to create an exchange, the Department of Health and Human Services (HHS) is required to develop and oversee it. At this point, it’s unclear whether HHS can get these exchanges up and running by the fall of 2013 so potential enrollees can select a plan. Of importance to clinical laboratories is that each participating insurance plan must cover certain “essential health benefits” as prescribed by the act. Included among the 10 required services is laboratory testing. The good news for laboratories is that implementing this provision should result in an increase in the volume of laboratory services ordered. CBO estimates that nearly 12 million people will obtain coverage through the exchanges in 2014, a number that is expected to increase to 27 million by 2018. Another feature of the healthcare reform law makes parents, children, and childless couples with family incomes be- low 133% of the federal poverty level eligible for Medicaid. More than half of the 30 million newly insured will receive coverage through the federal-state poverty program. This expanded coverage, in addition to increasing volume, may reduce the level of uncompensated care borne by healthcare providers, as well as increase the volume of testing for clinical laboratories. However, as with the exchanges, there are complications. Last summer, the Supreme Court ruled that states do not have to expand their Medicaid programs as specified in the healthcare law to continue participation in the program. Many states argue that Medicaid is already costly and that they cannot afford the expansion prescribed in the law. Currently, more than a dozen states, including Texas and Florida, which have large uninsured populations, have stated they will not participate in the Medicaid expansion. If these states maintain their opposition to this policy change, the benefits derived from healthcare reform, at least in the short-term, will be less than expected. Stay Informed and Prepare to Take Action Over the next 12 months, laboratory professionals will need to remain actively engaged in policy debates involving the federal budget and healthcare reform. Each of these issues could significantly affect laboratory operations in the short and long term as lawmakers seek to pull the U.S. back from the fiscal cliff and implement the most far-reaching federal health legislation since the passage of Medicare and Medicaid in 1965. AACC and CLN will continue to keep you abreast of these issues as decisions are made and announce opportunities for you CLN to influence policy. Vince Stine, PhD, is director of government affairs at AACC. Email: vstine@aacc.org Clinical Laboratory News January 2013 11 PATIENT SAFETY FOCUS T a k ing A im at R educing L ab E rr o rs Preventing Infections Related to Using Point-of-Care Testing Devices An Interview with Sharon M. Geaghan, MD The convenience and immediacy of point-of-care testing (POCT) has led to its use in many settings. But untrained or busy healthcare workers may overlook some basic sanitary practices when using POCT devices. This lack of attention is leading to nosocomial infections that can be attributed to contaminated devices. Here, Sharon M. Geaghan, MD, discusses how these infections occur and how to prevent them. Dr. Geaghan is professor of pathology and pediatrics at Stanford University School of Medicine in Palo Alto, Calif. Michael Astion, MD, PhD, chair of the Patient Safety Focus Editorial Advisory Board, conducted this interview. How would you sum up the problem related to POCT and nosocomial infections? Overall, I would say that there is a significant and pervasive lack of awareness about nosocomial infections associated with POCT, especially hepatitis B infections. In what settings do these infections occur? Typically, these infections occur during assisted monitoring of a POCT analyte. By assisted monitoring, I mean a POCT procedure performed by a nurse or other healthcare provider for the patient, rather than by the patient. Frequently, this involves a diabetic patient who is being helped with monitoring his or her blood glucose; however, any POCT device can transmit infection. These infections have been found in a variety of health care settings, including hospitals, ambulatory surgery centers, outpatient clinics, and assisted living facilities. What infectious agents are most likely to be transmitted by POCT devices? Hepatitis B and C are the most likely, although any blood-borne pathogen can be transmitted. For example, in one assisted living facility in North Carolina, officials discovered unsafe POCT practices that had significant consequences. Eight of 15 patients who had assisted POCT glucose monitoring contracted hepatitis B, and six died from the infection. While the severity of any infection transmitted in this manner can vary, this example illustrates that patients can suffer dire outcomes from POCT-transmitted nosocomial infections. How are these infections transmitted? There are several mechanisms, such as using a multi-lancet, finger-stick device on more than one patient; inadequately disinfecting and cleaning the POCT device between patients; placing meters in contaminated storage areas; and failing to change gloves and thoroughly wash hands between patients. It’s important to note that the highest risk for inadequate hand hygiene and glucose meter disinfection occurs when healthcare workers must assist large numbers of patients and only a few devices are available. As laboratorians know, POCT devices frequently become contaminated with blood. What many healthcare workers fail to recognize, however, is that transmission of infectious agents can occur even if no blood is visible on the finger-stick or POCT device. Recommendations for Reducing the Risk of POCT-related Infections ®Use finger-stick devices on only one patient. ®Use auto-disabling, single-use safety lancets for blood collection. ®Assign POC devices to a single patient whenever possible. ®Clean and disinfect POCT devices after every use if single-patient assignment is not possible. ®Change gloves between patients no matter what other precautions are being taken. Source: Reference 5 12 Clinical Laboratory News January 2013 Healthcare professionals need to take extra precautions in assisted living facilities to prevent transmitting infections from contaminated POCT devices. Do glucose meters have to be cleaned and disinfected after every use? Yes, especially if the meter is being used sequentially on different patients. In assisted living facilities, patients usually have dedicated meters. Even so, proper storage of the device is still important. It’s best to store the meter in the patient’s room, so that the risk of accidentally using the device on another patient and cross-contamination by contact with a blood-contaminated storage area or other equipment is eliminated. If the meter is reassigned to another patient, then it must be cleaned and disinfected. Healthcare workers may not be aware that meters can become contaminated in unsanitary storage areas. Even if the meter does not come in direct contact with patients, indirect transmission can occur via healthcare workers who handled the contaminated device. It’s also important to note that many institutions use alcohol wipes to disinfect devices. These wipes are not effective against hepatitis B virus present in dried blood. Instead, devices should be cleaned and disinfected with bleach-based disinfectants that are effective against hepatitis B and C. Can you summarize the recommendations to reduce the risk of POCT-related infections? The Food and Drug Administration and the Centers for Disease Control and Prevention have made some recommendations (See Table). The recommendations focus on cleaning and disinfecting instruments, proper hand hygiene, and implementing single-use finger-stick devices whenever possible. Assigning POCT instruments to a single patient is recommended as a best practice. References 1.Louie RF, Lau MJ, Lee JH, et al. Multicenter study of the prevalence of blood contamination on point of care glucose meters and recommendations for controlling contamination. Point Care 2005;4:158–63. 2.CDC. Notes from the field: deaths from acute hepatitis B virus infection associated with assisted blood glucose monitoring in an assisted living facility—North Carolina, August–October 2010. MMWR Morb Mortal Wkly Rep 2011;60:182. 3.CDC. Multiple outbreaks of hepatitis B virus infection related to assisted monitoring of blood glucose among residents of assisted living facilities—Virginia, 2009– 2011. MMWR Morb Mortal Wkly Rep 2012;61:339–43. 4.Thompson ND, Schaefer MK. “Never Events”: hepatitis B outbreaks and patient notifications resulting from unsafe practices during assisted monitoring of blood glucose, 2009–2010. J Diabetes Sci Technol 2011;5:1396–402. 5.FDA Patient Safety News. Preventing infections while monitoring glucose. www.accessdata.fda.gov/psn/printer-full. cfm?id=164 (Accessed November 2012). Failure to Follow Up on Test Results Failing to follow up on test results is a major problem in healthcare that contributes to unsafe patient care, particularly in ambulatory settings. In fact, almost a quarter of all medical errors occurring in outpatient settings can be attributed to poor followup of abnormal test results and are believed to represent 25% of malpractice lawsuits involving failures or delays in diagnosis. In the October 2012 issue of CLN’s Patient Safety Focus, I asked readers to respond to the following question: How does your lab ensure that pending lab results get to providers after a patient is discharged? Jaime Noguez, PhD, a clinical chemistry fellow at Emory University, Atlanta, provides her healthcare system’s information technology (IT) approach to this communication problem, as well as that used by Brigham and Women’s Hospital in Boston. Corinne Fantz, PhD Patient Safety Editorial Board Member No News Is Not Always Good News How IT Solutions Can Help Avert the Failures By Jaime Noguez, PhD Clinical laboratorians have primarily focused their efforts on reducing errors in the analytical phase of testing. Today, this phase of the total testing process is one of the most reliable systems in healthcare. However, the measure of success in laboratory medicine is based on all phases of the total testing process, from test ordering to appropriately interpreting and reacting to results. Because the clinical laboratory has historically been at the forefront of quality improvement activities in healthcare, we are well positioned to take on the next challenge for laboratory medicine: helping clinicians and other healthcare providers tackle the errors in the “extra-analytic” phases of the total testing cycle (Figure 1) (1). Test Result Management Systems In 2008, Emory University Hospitals implemented a unique system to improve the emergency department’s (ED) management of test results. Known as aftER Care, the system consists of an automated information service that enables clinicians and other qualified healthcare providers to manage results by automatically generating aftER Care events for every discharged patient with pending test results. These pending test results are usually for tests with long turnaround times, such as blood cultures or sexually transmitted diseases. The system populates these events into a list that must be reviewed by the end of each shift (Figure 2). Once the provider has acknowledged receipt of the test results, the event is signed electronically and cleared from the list. If the discharged patient cannot be reached by phone after several attempts, the system generates a letter using the address on file and it is sent to the patient via certified mail. Because patients in the ED sometimes provide incorrect contact information, the discharge documentation given to patients includes a phone number they can call to follow up on test results or with other questions regarding their treatment plans. Table 1 Overcoming IT Problems in Electronic Results Management Systems IT Problem Patient Safety Net Test result delivery. Track acknowledgement of test result delivery and report failures to responsible party. Data organized in departmental silos. Integrate reports, including pharmacy changes linked to pertinent lab results. Too many general alerts leading to alert fatigue. Design alerts to fit in clinician workflow and use them sparingly. The Consequences of Failure to Follow Up on Lab Results An Illustrative Case A 22-year-old woman presented to the clinic with complaints of headache and a low-grade fever for the past 3 days. The physical examination was unremarkable and the patient’s only other complaint when further questioned was intermittent abdominal pain that she attributed to pending menstruation. The physician ordered routine blood work and urinalysis with microscopic exam. Her blood tests were normal, and urinalysis results did not indicate a urinary tract infection. She was discharged and informed that she would be notified of additional pending test results. About 10 days later, the woman’s abdominal pain had become constant and she began experiencing dysuria, so she decided to go to the emergency department (ED). The ED physician ordered the same tests, but when he reviewed the results in her chart, he noticed that the patient had a positive urine PCR test for Chlamydia trachomatis. He was surprised to see that the result had been entered almost a week ago, but that it was never acknowledged in the patient’s medical record or acted upon by the attending physician. Before starting the woman on antibiotics, the physician performed a pelvic exam that revealed evidence of inflammation of the reproductive organs, possibly due to pelvic inflammatory disease. A pelvic ultrasound revealed thickened, fluid-filled fallopian tubes supporting the diagnosis of upper genital tract inflammation. ED chief nurse practitioner, Antionette Ward, NP, DNP, believes the aftER Care program has made pending test result management much easier compared to the previous paper-based system by condensing and organizing these events into a single-file format that can be tracked easily. The system also has been benefi- Figure 1 Conceptual Framework of the Testing Process Test ordered Test implemented Pre-analytic Test performed Analytic Test results returned to clinician Clinician responds to test results and documents Patient notified of test results Post-analytic Patient monitored through follow-up cial as a tool to document follow-up and provide feedback to clinicians about their diagnostic successes and failures. Recent data indicate that aftER Care has allowed Emory to close the loop on 99.8% of test results. A similar system to address efficiency and reliability of test result follow-up has been developed and implemented at Brigham and Women’s Hospital in Boston. The Longitudinal Medical Record Results Manager allows clinicians to review all chemistry, microbiology, hematology, radiology, and pathology results and document electronically that the appropriate caregiver has received the results. A major benefit of this system is that it enables clinicians to review the results in the context of the patient’s previous findings, in addition to the individual’s clinical history and medication list. Clinical Laboratory News January 2013 13 A few fail-safe mechanisms have been built into this system, such as nightly e-mail reminders that are sent to providers if critical lab results have been filed, but not acknowledged, and the ability for users to allow colleagues to review test results on their behalf (2). Although more comprehensive studies evaluating the success of Results Manager are underway, a small, randomized trial found that the percentage of documented follow-ups to post-discharge microbiology culture results more than doubled (3). Figure 2 Summary Screen of aftER Care The Pros and Cons of IT Solutions Managing follow-up of test results is a complex process. Using IT clearly has the potential to reduce the number of missed results by ensuring a safer and more systematic process; however, implementing technological solutions alone will not solve the problem of inadequate test follow-up. Busy physicians are tasked with assimilating massive amounts of information dumped into the electronic medical record (EMR) by different departments, including the lab, the pharmacy, radiology, and others. This requires physicians to spend a considerable amount of time filtering the information provided by each department in order to create a clinical picture of the patient and to develop a diagnosis and treatment plan. Because this data assimilation process is so intensive and prone to error, patient care is safer when these data can be easily visualized in an organized manner. IT solutions fail, however, when the data are present but critical information is overlooked. A root cause of this type of failure is organizing data into separate silos in the EMR. For example, lab data on microbial resistance and pharmacy data related to medication and dosage filed in two separate places in the EMR may cause a physician to continue an inappropriate antibiotic treatment because he didn’t check both. Connecting these two sets of data is likely to result in a decrease in medical errors. Furthermore, overall utilization and quality of laboratory testing, pharmacotherapy, This screen allows healthcare providers to view all pending laboratory results and clear the event from the list after results have been acknowledged. and patient safety improves, as one study documented (4). IT also fails when tools intended to aid medical providers, such as alerts, actually hamper their efforts. For example, pop-up alerts can be beneficial to notify physicians that the test being ordered is potentially redundant. But poorly executed systems sometimes produce excessive alerts that can overburden busy physicians and result in what is commonly called alert fatigue. When this happens, physicians often ignore important messages (5). On the other hand, IT solutions can improve result management if they are properly integrated into healthcare providers’ workflow. For instance, high-priority alerts with clinically significant information like low glucose levels in a patient on high-dose Does laboratory result management tie your physicians down? insulin must be delivered on a timely basis so that providers have sufficient time to take action. Similarly, structured handoffs based on computerized checklists can help transmit clinically significant test results during shift changes (6, 7). tion to help clinicians develop a systematic method for test result follow-up using the same or similar strategies already used to effectively manage patient results within the laboratory. Sharing Knowledge of Systems 1.Hickner J, Graham DG, Elder NC, et al. Testing process errors and their harms and consequences reported from family medicine practices: A study of the American Academy of Family Physicians National Research Network. Qual Saf Health Care 2008;17:194–200. 2. Poon EG, Wang SJ, Gandhi TK, et al. Design and implementation of a comprehensive outpatient Results Manager. J Biomed Inform 2003;36:80–91. 3. Poon EG, El-Kareh R, Roy C, et al. Impact of automated alerts on follow-up of post-discharge microbiology results: A cluster randomized controlled trial. J Gen Intern Med 2012;27:1243–50. 4. Schiff GD, Klass D, Peterson J, et al. Linking laboratory and pharmacy: Opportunities for reducing errors and improving care. Arch Intern Med 2003;163:893–900. 5. Appold K. Alert fatigue: When too many alerts lose physicians’ attention. Clin Lab News 2010;36:19. 6.Grimm, E. Shift-to-shift communication: What can labs learn from NASA and other highly reliable organizations. Clin Lab News 2011;37:14. 7. Fantz, C. The checklist movement: Why laboratories should embrace memory aids. Clin Lab News 2011;37:18–19. Clinical laboratorians are well positioned to play a greater role in reducing test result follow-up errors. We are well versed in using information systems to flag and document abnormal test results and critical values. In addition, we have stringent protocols, strategic processes, and efficient workflow designs along with clear definitions of the responsibilities of each individual in the laboratory. Given this combination of skills and systems, we stand in a unique posi- References Jaime Noguez, PhD, is a clinical chemistry fellow at Emory University, Atlanta, Ga. Email: jaime.noguez@ emory.edu. 14 Clinical Laboratory News January 2013 A Family Physician’s Perspective Laboratory Testing and Diagnostic Errors An Interview with Peter Weir, MD, MPH ARUP Laboratories’ workplace clinic serves roughly 4,500 employees, spouses, and dependents, and takes full responsibility for an individual’s healthcare, from mental and physical health to disease prevention and chronic disease management. Patients of the clinic include newborns, as well as patients over the age of 70. Seven health care practitioners— two family medicine physicians, four mid-level providers, three physician assistants, one nurse practitioner, and a half-time clinical pharmacist—attend to the needs of the entire patient population. Peter Weir, MD, MPH, is the medical director of the clinic and assistant clinical professor in the University of Utah Department of Family and Preventive Medicine in Salt Lake City. Here Dr. Weir gives his perspective on diagnostic errors. Brian Jackson, MD, MS, of the Patient Safety Focus Editorial Advisory Board, conducted this interview. Please describe your clinical responsibilities and practice setting. My role in the clinic is to see my own patient panel, as well as to supervise the medical care of all patients seen in our clinic. I’m fortunate to work with partners who are very competent and knowledgeable within their own area of expertise: pediatrics, women’s health, and sports medicine. So would you describe your clinic as a medical home, in other words a teambased healthcare delivery model that provides comprehensive and continuous medical care to patients with the goal of obtaining maximized health outcomes? Yes, that’s what we’re trying to accomplish. What are some of the challenges that you encounter in diagnostic testing? Every time I order a test of any kind, I’m very aware of the potential for ordering the wrong test for the patient’s condition, as well as the fact that even correctly ordered tests have weaknesses, for example false negatives and false positives. Ordering the incorrect test happens far more often than healthcare professionals probably realize. For example, in our clinic, many providers including me were ordering the serum H. pylori antibody test that gives very limited information. We later learned how much more appropriate the H. pylori breath test and stool antigen test are for determining if a patient has an active infection. Disruptive Behavior in the Laboratory At ARUP, we have an unusual patient population in that they tend to like being tested and often ask us to test for things that may not be clinically indicated. An example would be a patient who wants a test for a biomarker to screen for cancer even though the individual has no clinical indications. Ordering a cancer biomarker in this situation can lead to panic and a wild goose chase if the result comes back elevated. Another common problem is dealing with unexpected results. I’ve learned to repeat a lab or study if it doesn’t fit the clinical picture. Not infrequently, the repeat comes back with a normal result and avoids wasting a lot of time and resources. For example, I remember monitoring serial hemoglobins on a hospitalized patient with suspected upper gastrointestinal bleeding to determine if the patient would need an immediate transfusion and/or an urgent EGD to look for the source of bleeding. One hemoglobin result came back 50% lower than the previous one. I rushed to the patient’s bed expecting to find him very unstable and pale, yet he looked no different from when I last saw him. I ordered a CBC to confirm the result and then watched the phlebotomist as he drew the blood from a vein that the patient’s IV fluids were connected to. I realized in an instant that the last blood sample was diluted 1:1 with IV fluids, which led to the erroneous result. Do you encounter diagnostic testing issues that are specific to mid-level providers? I have found that inexperienced clinicians, not necessarily mid-level providers, tend to over-order tests when they are uncomfortable with a clinical situation. The problem they run into, however, is the more tests that are ordered, the more interpretation of results that is needed. For example, consider a patient with unusual joint pain. A less experienced provider might begin the workup with a panel of rheumatologic lab tests rather than carefully taking a history, doing a complete physical exam, and then targeting the appropriate laboratory work-up. Ordering panels of lab tests that are not well thought-out can generate misleading, and sometimes conflicting, results, and leads to confusion, unnecessary referrals, and patient anxiety. Can you think of other examples of patient harm resulting from diagnostic testing? The one that first comes to mind is the PSA test. I have followed the prostate cancer screening controversy for the past 12 years. In the late 1990s, many of us were concerned that there was no data to prove that mortality rates were decreasing despite the huge increase in prostate cancer detection. The worry was that we were catching a whole bunch of very slow, indolent tumors that probably would never have caused any clinical manifestation. In the last few years, large randomized controlled trials have confirmed that fear: PSA screening in asymptomatic men has not significantly reduced mortality. I am now reluctant to order PSA screening in men for fear that I will be doing the patient more harm than good. If you had a magic wand to wave over the clinical laboratory, what would you change? I would somehow bring the expertise from the clinical laboratory into our clinic. I am surrounded by physicians and scientists who have an exceptional knowledge base that I wish I could tap into at the point-ofcare. No doubt, every provider has limits to his/her own knowledge, and collaboration with colleagues often leads to better care for patients. An Invitation to Readers How has your lab improved patient safety? Send your success stories to me for inclusion in a future issue of Patient Safety Focus. Michael Astion, MD, PhD micheal.astion@seattlechildrens.org “Here is what I think of all your vitamin D tests!!!” Key Point: Disruptive behavior adversely affects laboratory quality and teamwork and can decrease the safety of our patients. References Astion ML. Disruptive behavior: How labs can recognize and overcome its negative effects. Clin Lab News 2011;37(7):16. Hernandez J. Confronting conflict in the lab: How lab managers can curb the effects of disruptive behavior. Clin Lab News 2011;37(7):15. Patient Safety Focus Editorial Board Chair Michael Astion, MD, PhD Seattle Children’s Hospital Seattle, Wash. Members Peggy A. Ahlin, BS, MT(ASCP) Consultant Salt Lake City, Utah Corinne Fantz, PhD Emory University Atlanta, Ga. James S. Hernandez, MD, MS Mayo Clinic Arizona Scottsdale and Phoenix, Ariz. Brian R. Jackson, MD, MS ARUP Laboratories Salt Lake City, Utah Clinical Laboratory News January 2013 15 profiles PT Referral Legislation Signed Into Law I r e g u l at o r y Supreme Court to Take Up Gene Patent Case A fter bouncing back and forth between appeals courts and the U.S. Supreme Court for almost 4 years, the Supreme Court has finally agreed to rule on the patentability of genes associated with breast and ovarian cancer, a decision that could have far-reaching effects on the business of molecular diagnostics. The American Civil Liberties Union and the Association for Molecular Pathology (AMP) originally filed the lawsuit in 2009 against the U.S. Patent and Trademark Office, as well as Myriad Genetics and the University of Utah Research Foundation, which hold the patents on the BRCA1 and BRCA2 genes. The lawsuit charges that patents on human genes violate the First Amendment and patent law because genes are “products of nature” and therefore cannot be patented. K-ASSAY ® The first time a decision was handed down on the case was in March 2010, when a New York federal court ruled the patents invalid. Myriad appealed, and a year later, the Court of Appeals for the Federal Circuit ruled that companies can obtain patents on the genes but cannot patent methods to compare those gene sequences. The case made its way to the Supreme Court for the first time in March 2012, when the court vacated the decision of the appeals court and instructed the court to reconsider the case in light of Mayo v. Prometheus, a Supreme Court decision unanimously invalidating patents on methods for evaluating a patient’s response to a drug. However, the appeals court essentially repeated its original ruling. Finally, in September 2012, the plaintiffs again asked the Supreme Court to rule on the patentability of genes, and the court agreed to hear the case. More information is available from AMP, www.amp.org. The Assay You Can Trust... Immunoassay Reagents for Chemistry Analyzers™ • Insulin Now it is possible to run quantitative insulin assays on your existing chemistry analyzer without the hazardous radioactivity of RIAs or the high cost of ELISAs. The insulin assay is highly specific and features liquidstable reagents requiring no dilution or mixing. 1 - 100 µIU/mL serum or plasma Assay Range: Sample Type: • α-1 Microglobulin Assay Range: 1.0 - 137 mg/L (serum / plasma) 0.2 - 34 mg/L (urine) • Fructosamine Colorimetric assay. Includes calibrator. Assay Range: 1 - 10 mmol/L • Direct Hemoglobin A1c Non-enzymic assay. No patient fasting required. On-board lysis step on many analyzers. Assay Range: 2 - 16% Adaptable to most chemistry analyzers (including Abbott Aeroset ®, Bayer Advia®, Beckman Synchron CX ® and LX ®, Dade Dimension ®, Roche / Hitachi, Olympus ® AU ™) For in vitro diagnostic use. KAMIYA BIOMEDICAL COMPANY 12779 Gateway Drive, Seattle, WA 98168 800-526-4925 206-575-8068 FAX: 206-575-8094 www.k-assay.com 16 Clinical Laboratory News January 2013 2011.09 CLN Insulin.indd 1 n a welcome victory for the lab community, President Obama on December 4 signed the Taking Essential Steps for Testing (TEST) Act, easing automatic penalties for labs that refer a proficiency testing (PT) specimen unintentionally. Under the existing law, a lab that referred a PT specimen to an outside laboratory could lose its CLIA certificate for 2 years, even if the specimen was not referred to deliberately cheat on a PT challenge. Since many labs have policies that automatically refer certain specimens to another lab, the statute has worried labs for years, although harsh punishments have been rare. If CMS discovered a PT referral, the sanctions under previous versions of the law would also have extended to the lab director and even the hospital in which a lab operated, potentially forcing a hospital to find another organization to perform all lab testing. With the changes to the law prescribed in the TEST act, CMS will now have legal leeway on how to punish a guilty lab, such as levying fines instead of revoking the lab’s CLIA certificate. The TEST act also makes clear that CMS can differentiate between a PT referral that happened through a clerical error in good faith, or through the lab knowingly trying to subvert the regulation. House Representatives Michael Grimm (R-N.Y.) and Michael Burgess (R-Texas) championed the act along with 13 cosponsors. In the Senate, Senator Amy Klobuchar (D-Minn.) introduced the bill with six cosponsors. The new legislation is posted on the Library of Congress’s THOMAS website, http://thomas.loc.gov. Medicare Pays First, Asks Questions Later R ather than make sure bills are correct before paying them, Medicare relies too much on after-the-fact audits, and as a result, wastes money, according to a recent Government Accountability Office (GAO) report. While a limited number of so-called prepayment edits saved Medicare $1.76 billion in 2010, the Centers for Medicare and Medicaid Services (CMS) could save more if the agency employed this approach more widely. In its investigation, GAO illustrated this point using analysis of certain Medicare coverage policies that govern in what cases or how frequently a test or procedure can be reimbursed. GAO identified $114.7 million in payments made in 2010 that appeared to be inconsistent with national and local policies and which could have been prevented through automated prepayment edits. Even when Medicare does use prepayment edits to screen bills from hospitals and other providers, Medicare’s system often fails, the report found. For example, GAO found that Medicare paid $8.6 million for claims that exceeded limits on the quantity of certain services that can be provided to a beneficiary by the same provider on a single date of service. In the GAO investigation, although CMS had ostensibly implemented prepayment edits to 8/5/2011 5:19:18 PM limit service quantities, a weakness in the agency’s systems allowed providers to bypass the quantity limits. The report noted that CMS most recently reported an improper payment rate of 8.6%—or nearly $30 billion—for 2011. More information is available from www.gao.gov. Warnings of EHR Incentive Abuses as Adoption Rises A ccording to a new report from the Medicare watchdog agency, the Office of the Inspector General (OIG), the Centers for Medicare and Medicaid Services (CMS) faces some serious holes in its system meant to reward physicians and hospitals that adopt electronic health records (EHRs). As physicians and labs have rushed to adapt to burgeoning networks of EHRs, CMS often does not go far enough to make sure that it only offers incentives to organizations that truly have the required technology, OIG found. CMS expects to give close to $7 billion in incentive payments between 2011 and 2016. OIG emphasized that because professionals and hospitals self-report data to demonstrate that they meet EHR incentive program requirements, CMS needs to verify that this data will help ensure the integrity of Medicare EHR incentive payments. OIG is recommending that CMS in at least some cases ask for proof from physicians and hospitals that their EHR systems measure up before paying, as well as issue better guidance to organizations on how to demonstrate compliance. CMS reviewed OIG’s report before publication, and disagreed with the first recommendation for stepped-up prepayment review. CMS believes requiring more documentation from providers up front could prove burdensome for physician offices and hospitals and delay payments. The full report is available online, http://oig.hhs.gov. Physician Adoption of EHRs Continues Rapid Growth I n related news, a December 2012 report from the National Center for Health Statistics (NCHS) found that 72% of officebased physicians used some kind of EHR system in 2012, a 26% increase over 2011. However, fewer physicians report using EHRs that meet certain minimal requirements suggested by the government, such as the ability to view lab results electronically. In 2012, 40% of physician offices had such systems, up from 34% in 2011. The NCHS report also found that EHR adoption varied by state. In 2012, the percentage of physicians using any EHR system ranged from 54% in New Jersey to 89% in Massachusetts, and the percentage having a system that met the criteria for a basic system had an even wider range: from 22% in the District of Columbia to 71% in Wisconsin. Notably, 66% of all office-based physicians surveyed reported that they planned to apply, or already had applied, for the EHR incentive program. The NCHS report is available through the Centers for Disease Control and Prevention website, www.cdc.gov/nchs. profiles industry D ioGenix has entered a sponsored research agreement with Fast Forward, a subsidiary of the National Multiple Sclerosis Society, to develop a novel blood test for multiple sclerosis (MS). DioGenix’s current MS diagnostic, MSPrecise, uses nextgeneration sequencing technology to detect changes to the adaptive immune system. It accomplishes this by measuring mutations found in rearranged immunoglobulin genes in B cells isolated from cerebral spinal fluid. Under the terms of the agreement, DioGenix will receive up to $500,000 of funding from Fast Forward to determine if MSPrecise’s approach also works with blood samples. If it does, it could give clinicians the ability to diagnose MS earlier and to distinguish MS from other similar immune-mediated neurological diseases. Roche Breaks Ground on New Customer Training Center will allow Insight to continue developing lung cancer companion diagnostics. The first, a Phase II contract, will give Insight $1.5 million to further validate its Insight ALK Screen, a real-time qPCR-based test designed to identify the approximately 5–10% of lung cancer patients with oncogenic anaplastic lymphoma kinase (ALK) mutations and fusions. Screening for these mutations is essential for determin- Quanterix Licenses Immunoassay Technology to bioMérieux Q uanterix and bioMérieux have signed a strategic agreement granting bioMérieux worldwide exclusive rights to Subscribe Today—It’s FREE! The easiest decision you’ll make today! R AACC is pleased to offer you a free subscription* to its highly regarded publication, Clinical Laboratory News. CardioDx Participates in Study of Heart Disease Diagnostics Considered by many to be the best at delivering hands-on, lab-focused information, Clinical Laboratory News is a must-read for everyone in the lab. oche has broken ground on a new Learning and Development Center at its North American headquarters in Indianapolis. The center will host the training of more than 1,500 customers each year, and is the first element of a $300 million site transformation intended to support the company’s growing diagnostics and diabetes care businesses. C ardioDx has joined the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE), the first large randomized trial to compare the effectiveness of anatomical versus functional noninvasive diagnostic tests for assessment of patients with suspected coronary artery disease (CAD). As a secondary goal, the trial will also evaluate the ability of CardioDx’s blood-based gene expression test, Corus CAD, to predict major clinical cardiovascular events. “The findings of PROMISE also will help us determine the potential for developing a new test specifically focused on prognosis for CAD patients, which could involve the use of next generation technologies to identify expression of genes predictive of future events,” said David Levison, CardioDx president and CEO. Researchers expect 10,000 patients to enroll in the study, which is sponsored by Duke University in collaboration with the National Heart Lung and Blood Institute. NCI Funds Insight Genetics’ Development of Lung Cancer Co-Diagnostics T he National Cancer Institute granted Insight Genetics two Small Business Innovation Research (SBIR) contracts that Quanterix’s Simoa platform. Simoa is an immunoassay technology with multiplex capability that measures single molecules and is highly sensitive for proteins that traditional analog-based methods cannot detect. Using this platform, bioMérieux plans to develop a menu of tests focused on infectious diseases. Quanterix also hopes that this alliance will give the company the “flexibility to leverage Simoa technology and apply it in other commercial opportunities of interest, whether that be in biomedical research, bioterrorism, blood banking, or point-of-care diagnostics,” said Paul Chapman, Quanterix president and CEO. Each month, our seasoned editorial team tackles the top current and emerging issues, from clinical decision-making and effective lab management to point-of-care testing and more. Now you can have Clinical Laboratory News sent directly to you every month! What decision could be easier? Clinical Laboratory News “ CLN is a must-read for everyone in the lab. “ DioGenix and Fast Forward Partner to Develop Multiple Sclerosis Dx ing when to prescribe ALK inhibitors. The second SBIR grant, a Phase I Fast Track contract of $200,000, will fund creation of a panel test for detecting RET and ROS1 fusions along with DEPDC1 expression in non-small cell lung cancer (NSCLC). With this funding, Insight Genetics hopes to create a high-throughput diagnostic panel that could identify these biomarkers, which account for up to 9% of NSCLC cases. To receive your free* subscription to Clinical Laboratory News, go to www.aacc.org and click on Publications, Clinical Laboratory News. *Free in the U.S. only. Subscribers outside the U.S. must pay a postal fee. 1850 K St, NW, Suite 625 Washington, DC 20006 1-800-892-1400 Clinical Laboratory News January 2013 17 profiles mance in detecting current or recently circulating virus strains. CDC also cautioned that respiratory specimens should be collected when influenza virus is at its peak, with 24–72 hours of symptom onset. diagnostic Rapid Flu Tests Have Variable Detection at Lower Virus Concentrations A n evaluation of 11 commercially available rapid influenza diagnostic tests (RIDT) by the U.S. Centers for Disease Control and Prevention (CDC) found that while most tests detected viral antigens in high-concentration samples, detection varied by test and viral subtype at lower concentrations (Morb Mortal Wkly Rep 2012 Nov 2;61:873–76). The results suggest that clinicians and laboratorians should use RIDTs cautiously for diagnostic treatment and infection control decisions in clinical settings. CDC conducted the study because there had not been a recent comprehensive analytical evaluation of RIDTs using a standard method and involving all 11 U.S. Food and Drug Administration-cleared RIDTs. In partnership with CDC, researchers at the K-ASSAY ® Medical College of Wisconsin prepared swab samples or mock nasal wash specimens from several dilutions of 16 stock influenza A and seven influenza B viruses provided by CDC. The stock viruses were all representative of those circulating in the U.S. since 2006. The researchers measured concentrations of the viruses’ nucleoproteins (NP) using isotope dilution tandem mass spectrometry, and the egg infectious dose per milliliter values were at least as high as those reported in human clinical specimens. The investigators performed three separate tests for each virus/RIDT combination, with positivity defined as two positive results out of the three. Although all RIDTs detected virus at the highest virus concentrations, some did not detect at subsequent dilutions. Since part of this variation could be due to the use of different antibodies in the various tests, CDC recommended that users periodically evaluate RIDT perfor- The Assay You Can Trust... Immunoassay Reagents for Chemistry Analyzers™ Lipoprotein Assays For use on most chemistry analyzers including: Abbott Aeroset / Architect , Beckman Synchron & Olympus AU, Horiba ABX Pentra, Mindray, Roche / Hitachi, Roche Cobas, Siemens / Bayer Advia & Dimension, and others. • Apolipoprotein AI • Lipoprotein(a) • Apolipoprotein B For in vitro diagnostic use. • Apolipoprotein AII • Apolipoprotein E • Apolipoprotein CII For research use only. Not for use in diagnostic procedures in the U.S. Laboratory Testing Service Now Available For: > Apo AII > Apo CIII > Apo CII > Apo E KAMIYA BIOMEDICAL COMPANY 800-526-4925 206-575-8068 FAX: 206-575-8094 www.k-assay.com 18 Clinical Laboratory News January 2013 2011.09 CLN Lipoprotein.indd 1 L ower baseline high-density lipoprotein cholesterol (HDL-C) level is a significant independent predictor of the development and progression of diabetic nephropathy, but not retinopathy, in type 2 diabetics (Diabetes Care 2012;35:2201–6). The results suggest that measuring HDL-C may be useful in tailoring screening and therapeutic strategies. The ADVANCE study involved 11,140 patients with type 2 diabetes who were at least 55 years old at the time of enrollment and had at least one other cardiovascular disease risk factor. Baseline lipid levels, HbA1c, and creatinine levels were determined, and creatinine and HDL-C levels were repeated at 24 and 48 months. Urine samples also were collected at baseline, 24, and 48 months for calculation of the albumin-to-creatinine ratio (ACR). Participants’ mean baseline HDL-C level was 1.3 mmol/L. Nearly one-third developed new or worsening microvascular disease during follow-up, with 28% experiencing a renal event and 6% a retinal event. Compared with subjects in the highest third of HDL-C values, those in the lowest third had a 17% adjusted increased risk of microvascular event. Patients in the lowest third of HDL-C levels also were more likely to maintain or progress to a worse category of urinary ACR over time, compared with those in the middle or upper thirds of HDL-C levels. In contrast, the researchers observed no association between baseline HDL-C levels and development of retinopathy or any specific type of retinal event. The findings suggest that there are differences between the pathophysiologies of the two types of microvascular disease. The authors called for further research to explore possible benefits of HDL-C-raising therapies in patients with type 2 diabetes. Fructosamine, Glycated Albumin, and 1,5-AG all Independent Predictors of Diabetes Risk F • Apolipoprotein CIII 12779 Gateway Drive, Seattle, WA 98168 HDL-C Predicts Diabetic Nephropathy but not Retinopathy ructosamine, glycated albumin, and 1,5-anhydroglucitol (1,5-AG) are strongly associated with subsequent development of diabetes, independent of baseline HbA1c and fasting glucose (Diabetes Care 2012;35:2265–70). The findings suggest that elevations in these analytes may be useful indicators of future diabetes risk. The researchers investigated this association because fructosamine, glycated albumin, and 1,5-AG all have been of recent interest in assessing glycemic control in patients for whom use of HbA1c is problematic, including those with anemia, hemolysis, or renal disease. However, little investigation has taken place in terms of their levels in nondiabetic populations or association with future development of diabetes. The study, a subset of the long-standing Artherosclerosis Risk in Communities 8/5/2011 5:15:25 PM study, involved 1,200 participants without an initial diagnosis of diabetes who were followed for a median of 3.3 years. During the study period, there were 119 new cases of diabetes. When compared with the lowest quartile, the highest quartile of baseline fructosamine and glycated albumin levels were significantly associated with diabetes risk, with hazard ratios of 3.99 and 5.22, respectively. These associations remained significant after adjustment for fasting glucose and HbA1c concentrations. Higher baseline quartiles of 1,5-AG were inversely associated with incident diabetes, and remained so after adjustment for diabetes risk factors and fasting glucose and HbA1c levels. The authors called for additional studies to evaluate more extensively the associations between all three markers and longterm complications of diabetes, as well as their potential clinical utility for monitoring glycemic control. Meta-analysis Supports Age-independent Definition and Staging of CKD A meta-analysis of 46 different cohorts involving more than 2 million participants found that both estimated glomerular filtration rate (eGFR) and high albuminuria were independently associated with mortality and end stage renal disease (ESRD) regardless of age across a wide range of populations (JAMA 2012;308: doi:10.1001/jama.2012.16817). The findings suggest that although clinicians might consider some variation in managing chronic kidney disease (CKD) based on age, cost, and benefits, with respect to risk of mortality and ESRD, a common definition and CKD staging based on eGFR and albuminuria regardless of age would be more appropriate. The authors conducted this analysis because there has been interest in using eGFR and albuminuria to define and stage CKD. However, controversy exists about whether age modifies their independent and combined associations with clinical risk The investigators found that risk of mortality and ESRD were higher at lower eGFR and higher albuminuria at every age category. Relative mortality risk for reduced eGFR decreased with increasing age. In the case of higher albuminuria, the reduction in relative risk with increasing age was less evident. In patients with CKD, the adjusted relative hazards of mortality did not decrease with age, and in all cohorts, the relative risk of ESRD associated with lower eGFR or higher albuminuria were comparable across age categories. The findings indicate that kidney measures used for defining and staging CKD are strong predictors of clinical risk across the full range of ages, including patients older than age 75. 2013 AACC Annual Meeting July 28–August 1 Houston, Texas news f ro m t h e fda CDRH Releases Proposed Guidance Development for FY 2013 Nanosphere Screen for CYP2C19 Mutations Receives FDA Nod DA’s Center for Devices and Radiological Health (CDRH) has released its list of priorities for developing guidance documents for 2013. These priorities include medical device guidance documents that the agency fully intends to publish within a year (the A-list), and guidance documents FDA will publish within the year as resources permit (the B-list). Guidance documents prioritized to the A-list include: In Vitro Companion Diagnostic Devices; Mobile Medical Applications; The 510(k) Program: Evaluating Substantial Equivalence in Premarket Notifications; and De Novo Classification Process (Evaluation of Automatic Class III Designation). Among the B-list items, CDRH included Direct to Consumer Genetic Testing: IVDs. Neither the A nor B list mention the long-awaited guidance document for lab-developed tests (LDTs). In July 2010, FDA announced it planned to require all LDTs to undergo regulatory review. The complete lists are available online, www.fda.gov/MedicalDevices/ DeviceRegulationandGuidance/Overview/ MDUFAIII/ucm321367.htm. DA has cleared Nanosphere’s CYP2C19 Nucleic Acid Test on the Verigene System. The test identifies variants in the gene for the cytochrome P450 2C19 (CYP2C19) enzyme that metabolizes approximately 15% of all prescribed drugs. Two variants in the CYP2C19 gene, *2 and *3, lead to reduced drug metabolism, while the *17 variant causes increased drug metabolism. Using wholeblood samples, the Verigene CYP2C19 Test detects the presence of these mutations in less than 2.5 hours, providing clinicians with essential guidance for treating patients with drugs metabolized by this pathway. F F index to advertisers Please visit these websites to learn more about the products in this issue. BD Biosciences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20 www.bdbiosciences.com/go/canto Cerilliant . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7 www.cerilliant.com Evergreen Scientific . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 www.evergreensci.com Kamiya Biomedical Company . . . . . . . . . . . . . . . . . . . . . . . . . . . 2, 16, 18 www.k-assay.com Zeus Scientific . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 www.zeusscientific.com/products/technology-systems/ifa Stay ahead of the curve. Essential Knowledge and Tools for Working in Today’s Clinical Lab Read CLN on your iPad. Get our FREE App. Go to the Apple App Store to download it today. April 28 – May 2, 2013 | La Jolla, California San Diego Marriott La Jolla Advance your skills from intermediate to cutting-edge. ✔ Learn about the latest in best practices, new technologies, and smart management tools ✔ Analyze case studies with renowned experts ✔ Expand your professional network ✔ Earn up to 40 continuing education credits Learn more and register at aacc.org/events from American Association for Clinical Chemistry • 1850 K Street, NW, Suite 625, Washington, DC 20006 • 800-892-1400 Clinical Laboratory News January 2013 19 The BD FACSCanto™ II Consistency you can depend on to keep work flowing. Reliability is timeless. With all the challenges facing clinical labs, one thing is clear: reliability is timeless. For over 40 years, BD has delivered flow cytometry systems that are progressively more powerful, more dependable, and easier to use. A lot has changed over that time, but the BD FACSCanto™ II continues to be a mainstay of core labs with proven reliability and consistency to help clinicians deliver patient care. BD, BD Logo and all other trademarks are property of Becton, Dickinson and Company. © 2012 BD 23-14766-00 Today’s BD FACSCanto II system is complemented by a full spectrum of BD conjugated antibody specificities including the BD Horizon™ V500-C violet reagents. You can count on BD to make your job easier, your workflow more efficient, and your results more reliable—today and tomorrow. For more information, go to: bdbiosciences.com/go/canto BD Biosciences 2350 Qume Drive San Jose, CA 95131 bdbiosciences.com
© Copyright 2025